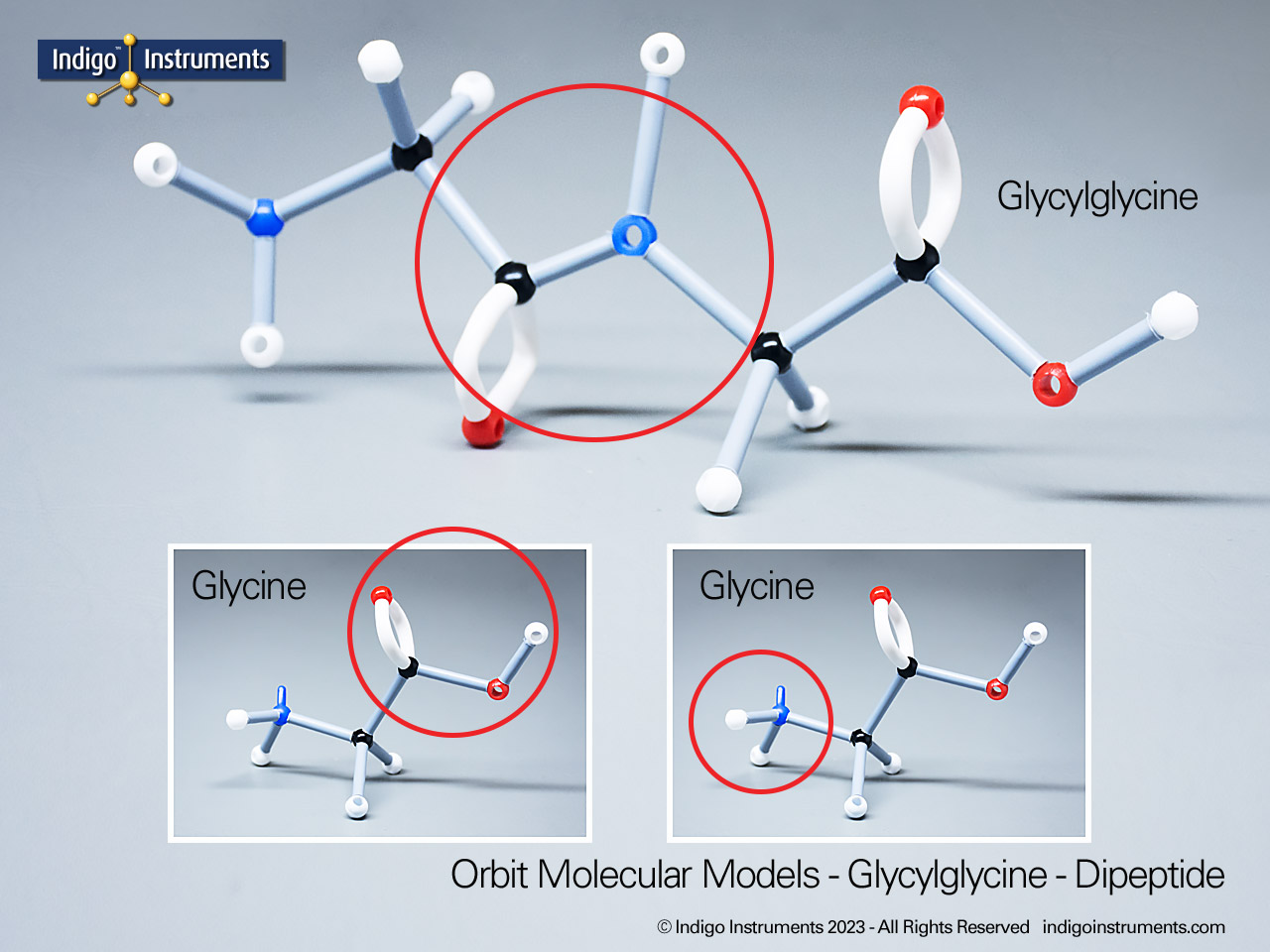
Alkyne Hydrocarbon Structure Models
SKU: 68845NV
Explore alkyne molecular models for teaching organic chemistry and biology. Learn about triple bonds, IUPAC naming, reactivity, and real-world uses in medicine and materials science.
Alkyne molecular models are essential for visualizing hydrocarbons with carbon–carbon triple bonds, a defining feature of this functional group. These models help students understand linear geometry, bond hybridization (sp), and the unique reactivity patterns that distinguish alkynes from alkenes and alkanes. By physically manipulating molecular models, learners can better grasp concepts such as naming according to IUPAC rules, functional group transformations, and the role of alkynes in synthetic chemistry, pharmaceutical design, and biochemical processes.
What Different Students Need to Know About the Alkyne Functional Group
| Aspect | Chemistry Majors | Biology / Medicine / Nursing Majors |
|---|---|---|
| Structural Understanding | Precise visualization of carbon–carbon triple bonds, linear geometry, and bond lengths. | Basic recognition of triple bonds and how they affect molecular shape and biological activity. |
| Nomenclature | Detailed IUPAC naming, including position numbering and substituent priority. | Familiarity with simple alkyne names in pharmacology and biochemistry contexts. |
| Reactivity & Mechanisms | Mechanistic understanding of electrophilic addition, nucleophilic attack, and acidity of terminal alkynes. | Awareness of alkyne reactivity in metabolic pathways and drug metabolism. |
| Applications | Use in polymer synthesis, advanced materials, and complex organic synthesis. | Use in medicinal chemistry, diagnostic agents, and potential therapeutic targets. |
| Laboratory Skills | Proficiency in synthesis, purification, and characterization of alkynes. | Understanding safe handling and relevance of alkynes in physiological conditions. |
Important Learning Outcomes
- Chemistry Majors Should Be Able to:
Master IUPAC rules for naming alkynes and identifying triple bond position.
Understand sp hybridization and its effect on bond length and bond angle.
Predict and explain reactivity in hydration, halogenation, hydrogenation, and oxidative cleavage reactions.
Recognize the acidity of terminal alkynes and applications in organic synthesis.
- Biology / Medicine / Nursing Majors Should Be Able to:
Recognize triple bonds in pharmacologically active molecules.
Understand how linear geometry influences biological interactions.
Appreciate the role of alkyne derivatives in drug development and diagnostics.
Relate alkyne-containing compounds to metabolic and physiological processes.
Features & Benefits of the Orbit Framework Student Organic Chemistry Molecular Model Kit 68845NV for Teaching Alkynes
| Feature | Benefit |
|---|---|
| Accurate linear geometry for sp-hybridized carbons | Visually enforces 180° bond angles of alkynes, helping students predict molecular shape and linear connectivity in reactions and assemblies. |
| Distinct triple-bond connector (rigid, tactile piece) | Makes the presence of a triple bond obvious and allows learners to compare single, double, and triple bond rigidity and rotation constraints. |
| Colored segments for σ and π components | Clarifies the concept of a σ framework plus two π systems—ideal for introducing orbital overlap and reactivity differences versus alkenes and alkanes. |
| Terminal-alkyne proton marker | Highlights acidity of terminal alkynes (pKa discussion) and supports demos of deprotonation, acetylide formation, and subsequent nucleophilic coupling. |
| Replaceable reaction accessory pieces (H?, Lindlar, Na/NH?) | Enables hands-on demonstration of partial/full hydrogenation and stereochemical outcomes (cis vs trans) without wet chemistry. |
| Modules for regioselectivity & addition reactions | Teach Markovnikov vs anti-Markovnikov outcomes, hydrohalogenation, and halogenation with clear, buildable examples. |
| Buildable polymerization sequences | Showcases how alkynes can participate in polymer chemistry (e.g., alkyne metathesis, click-type couplings) and link to materials discussions. |
| Compatibility with Orbit functional-group kits | Integrates easily into multi-step reaction demonstrations (e.g., alkyne → alkene → alkane or alkyne → acetylide → coupling) for curriculum continuity. |
| Durable classroom-grade construction | Stands up to repeated handling, making it suitable for lectures, labs, and outreach without loss of fit or function. |
| Instructor guides & assessment cards included | Provides stepwise activities, quiz prompts, and challenge builds to test understanding of hybridization, acidity, stereochemistry, and mechanism prediction. |
Indigo Instruments has held inventory of genuine Cochranes of Oxford (Orbit) atoms & bonds for 30+ years. These parts are compatible with every molecular model set we have sold since day 1. This quality may appear expensive but no parts support from other vendors costs even more.