Alcohol Functional Group Chemical Structure
SKU: 68845NV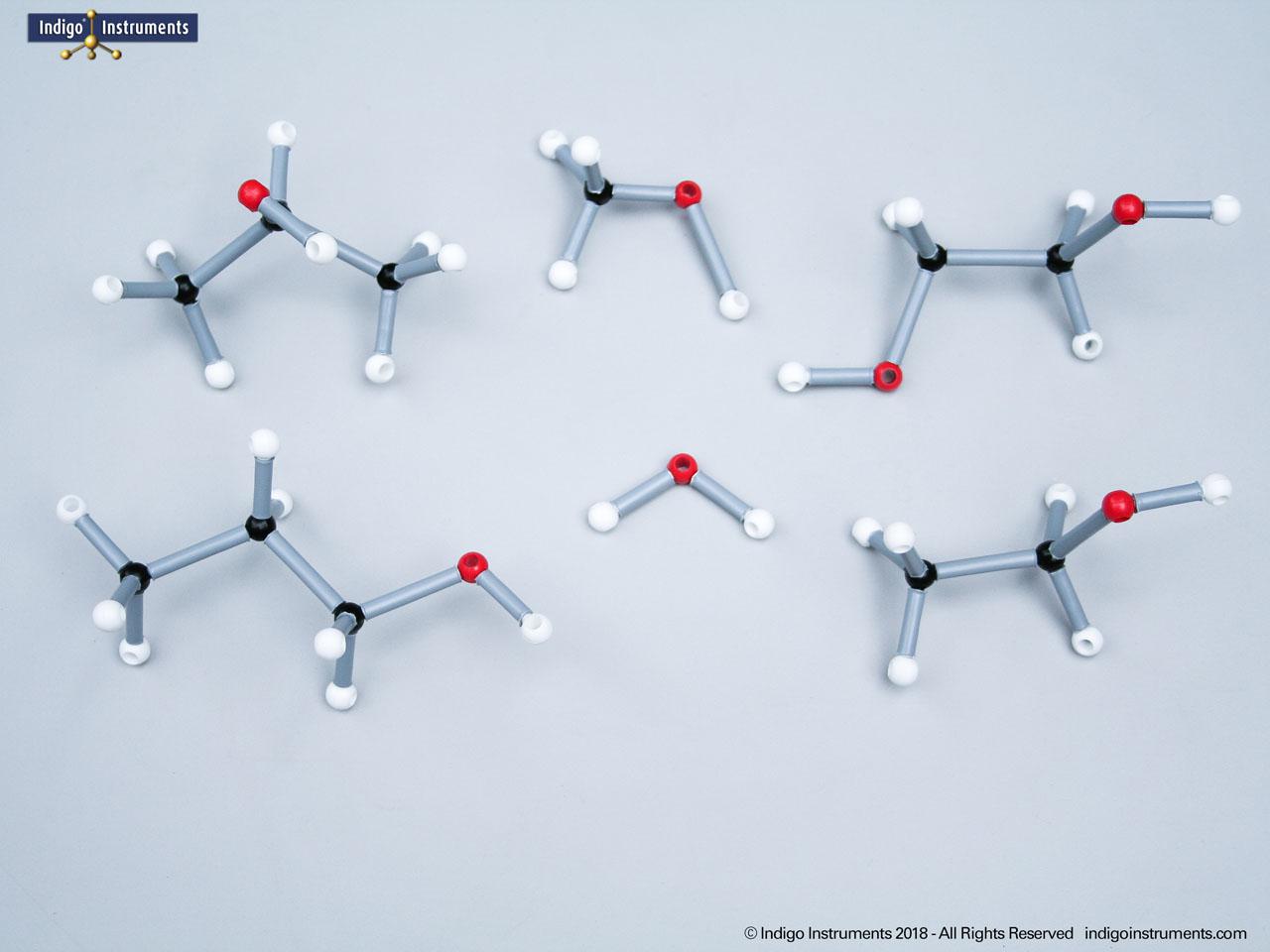
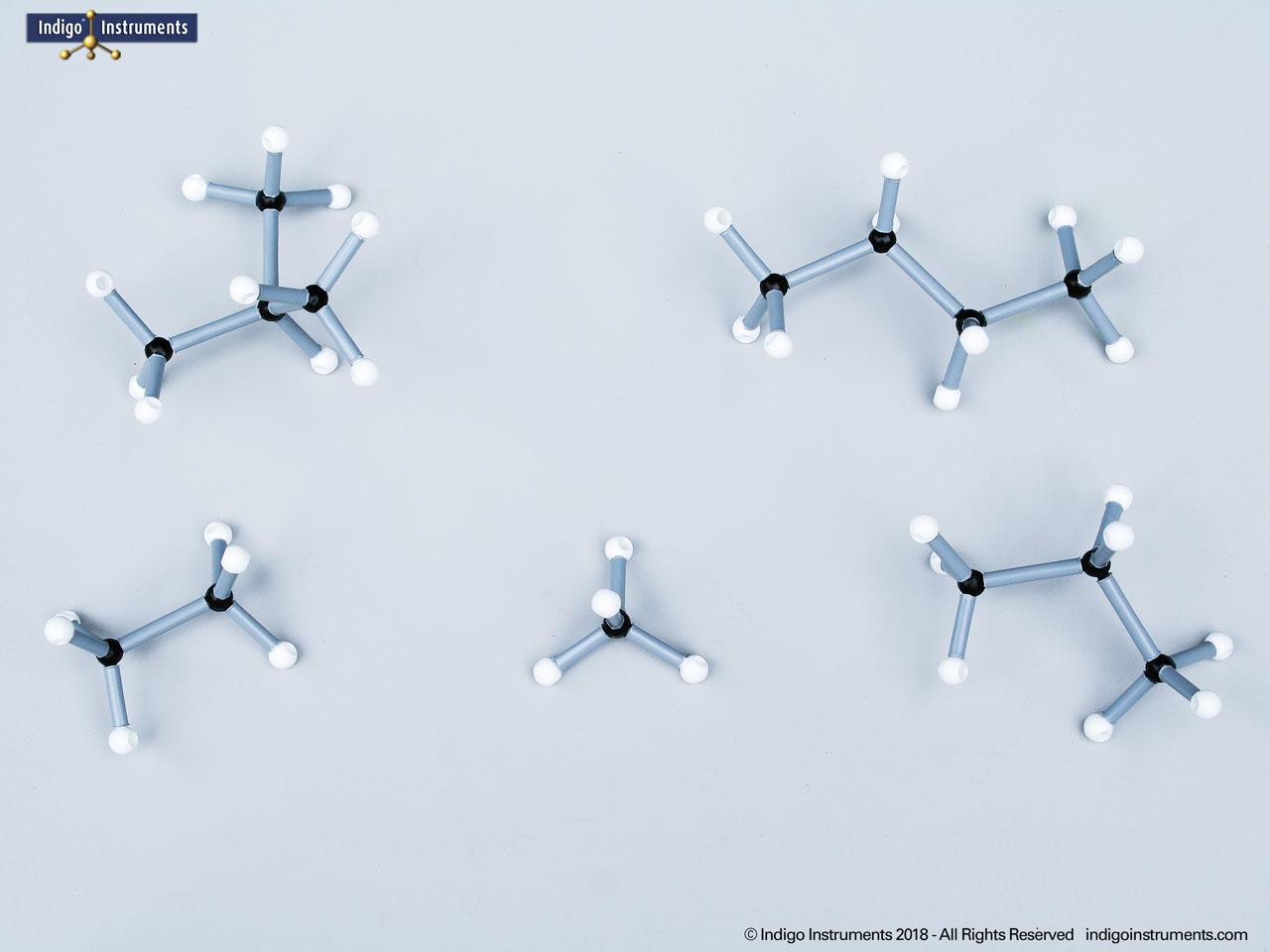
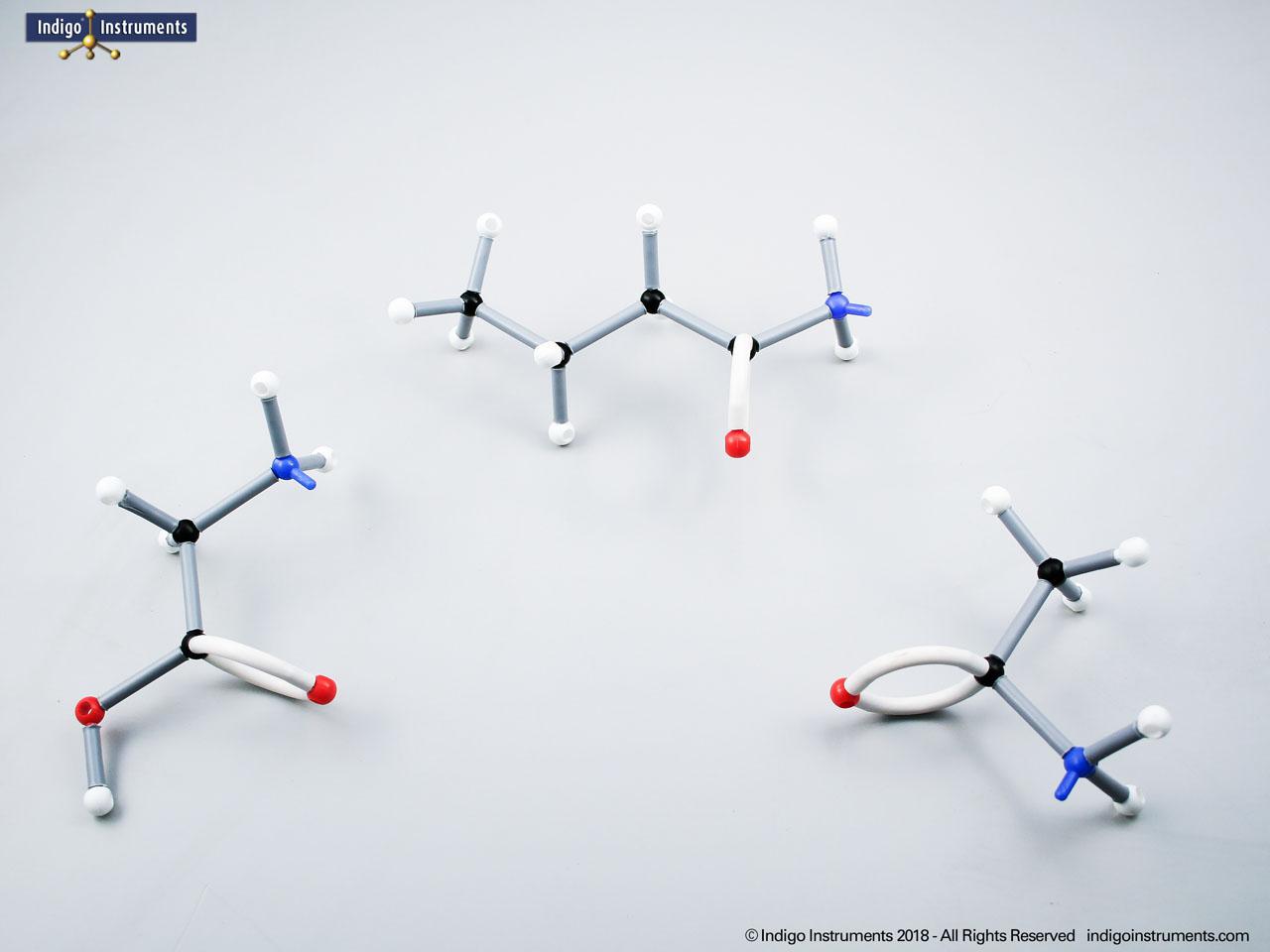
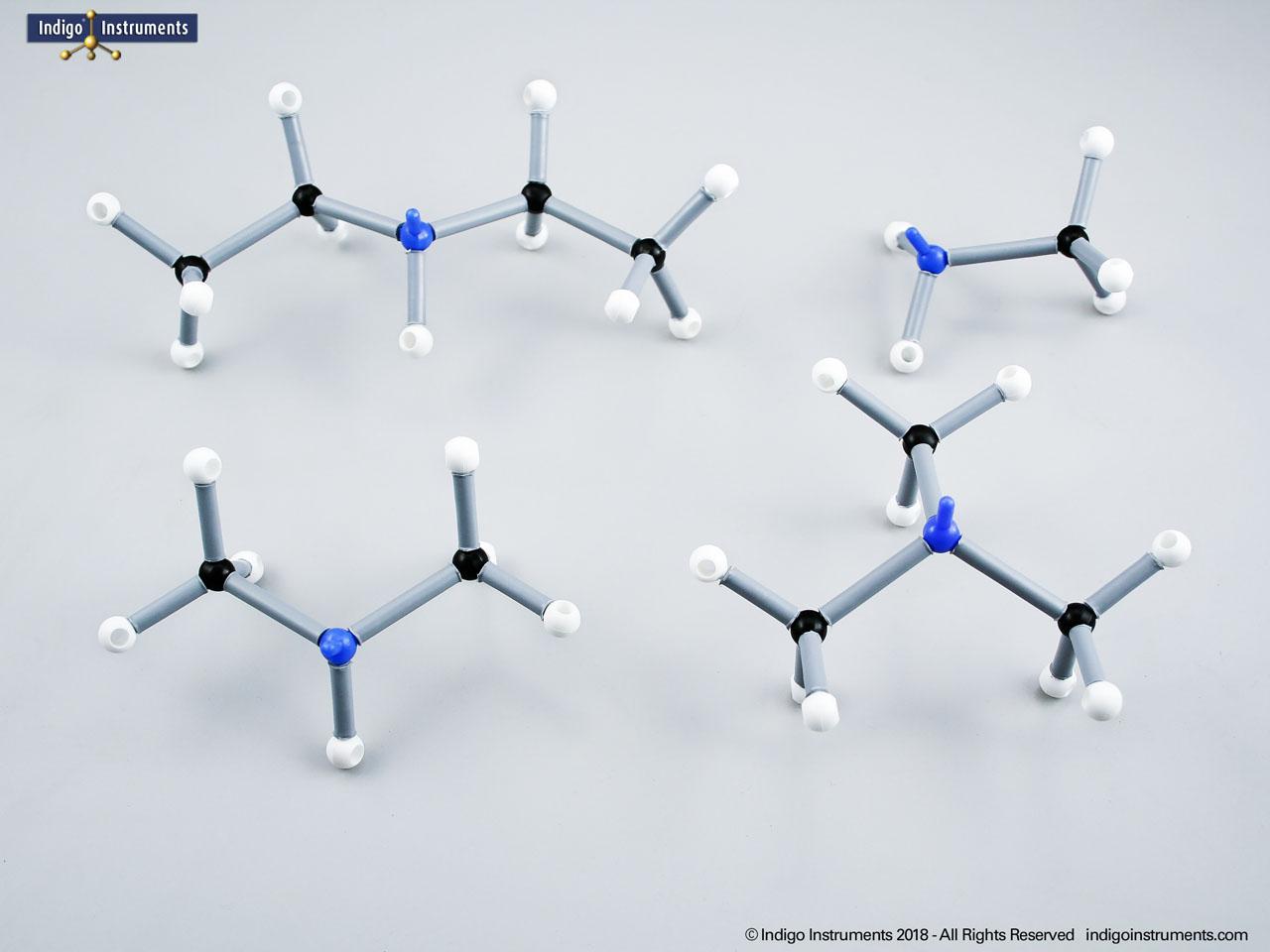
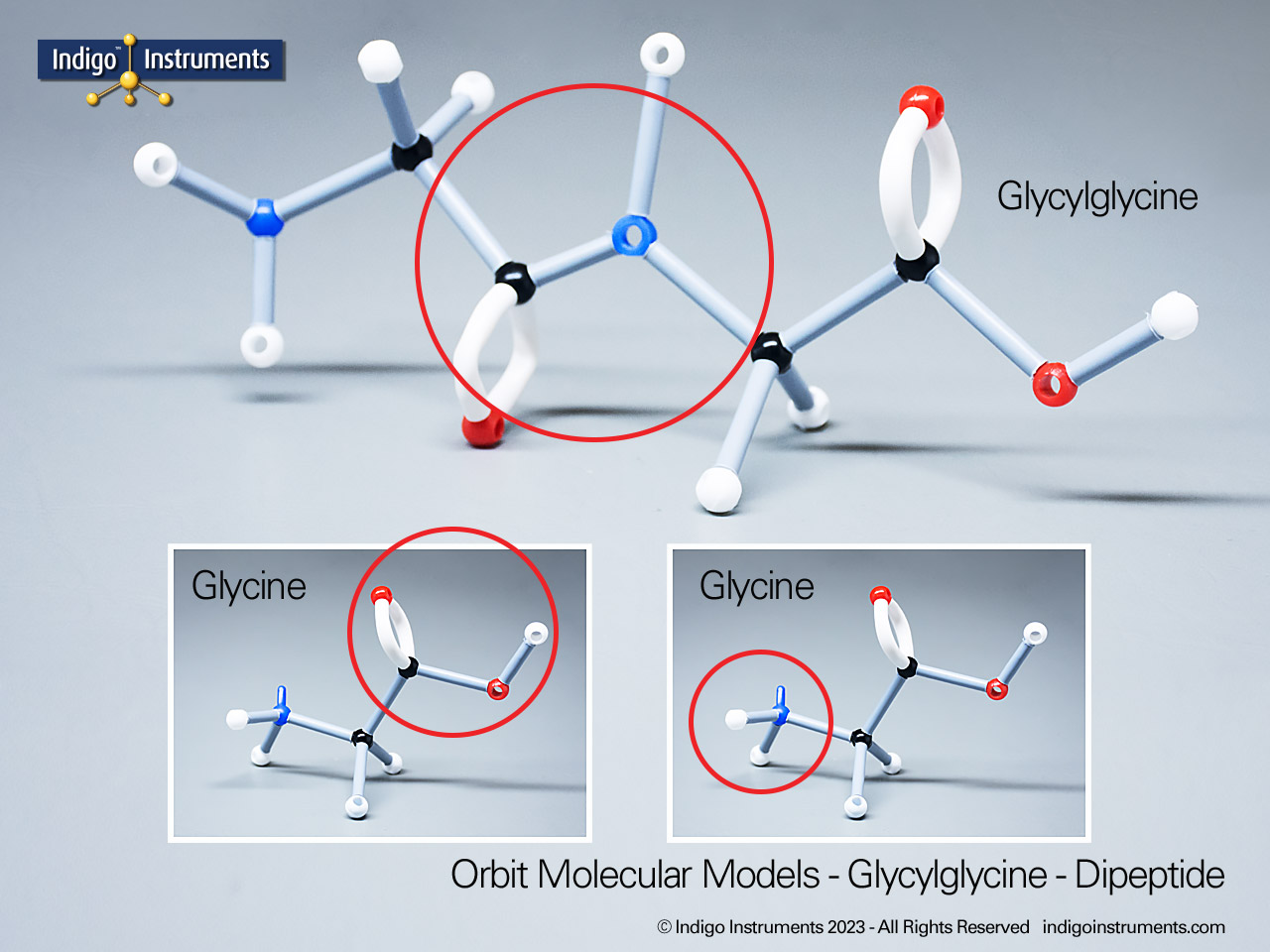
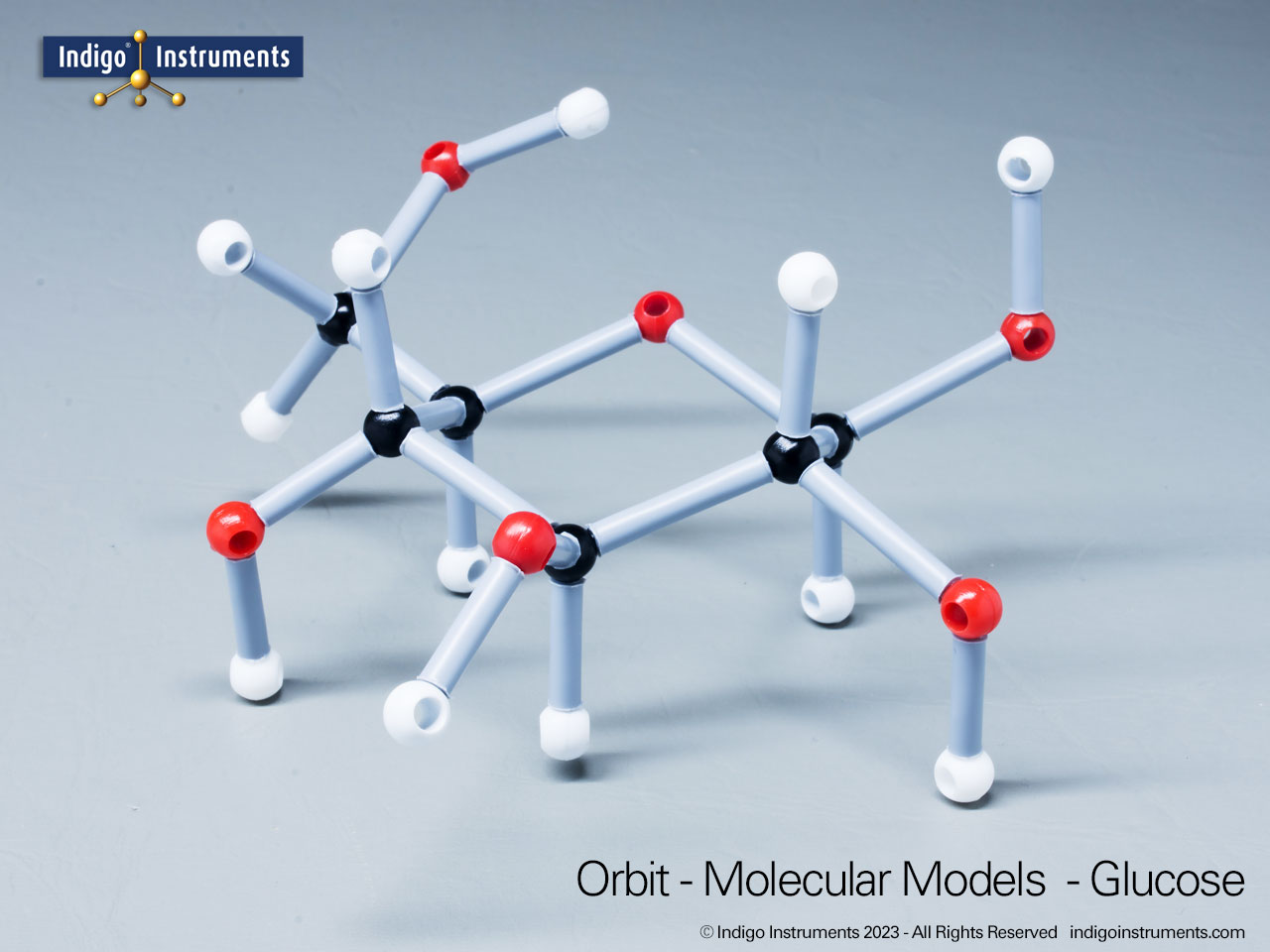
Chemistry and biology students studying hydroxyl group structures in sugars, amino acids, & lipids can build, visualize, and teach the alcohol functional group with this Orbit molecular model set.
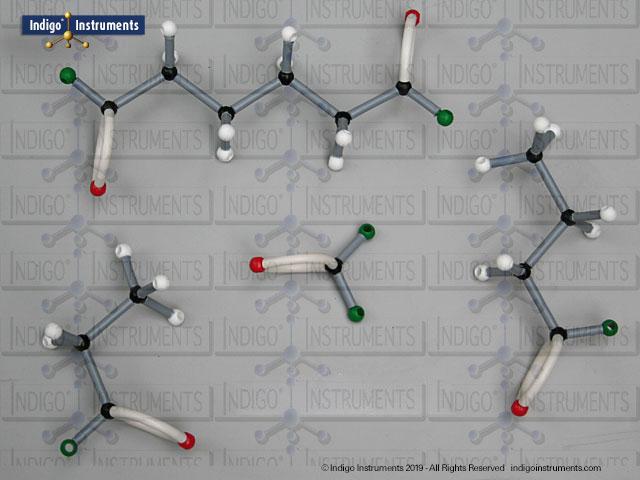
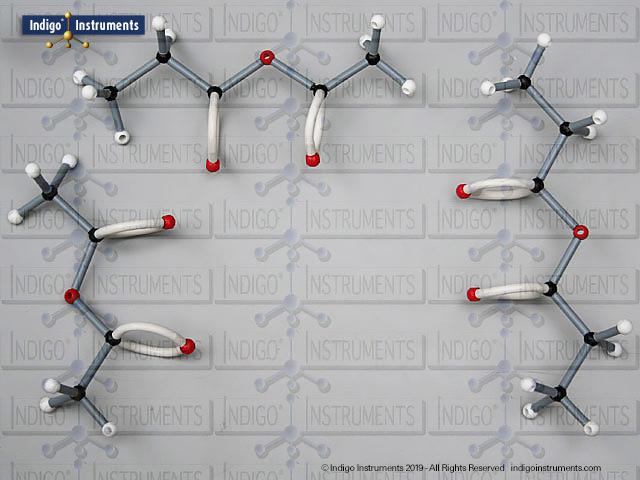
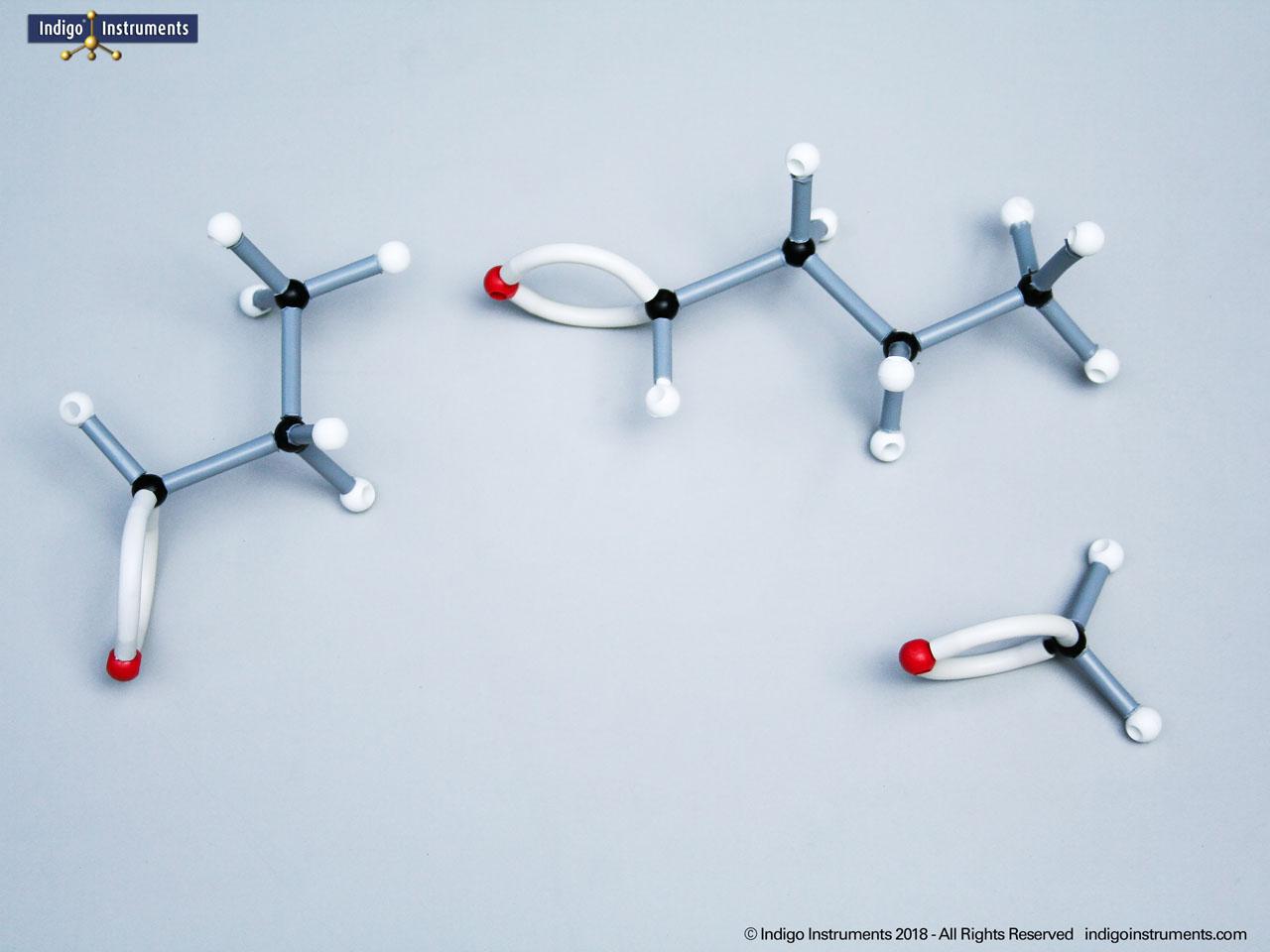
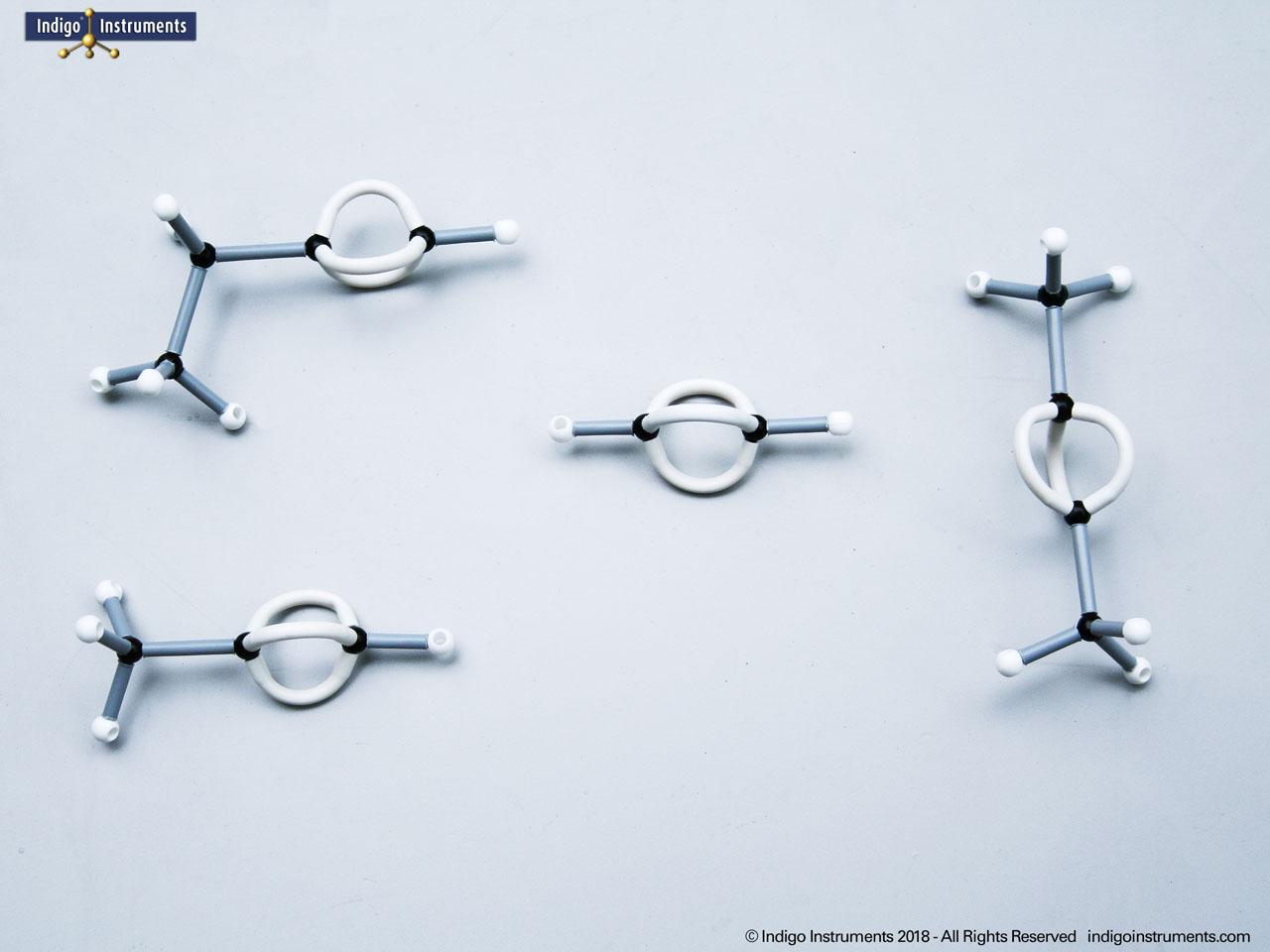
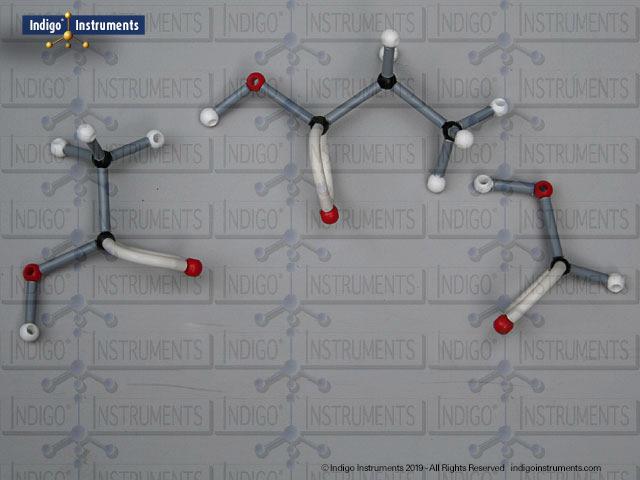
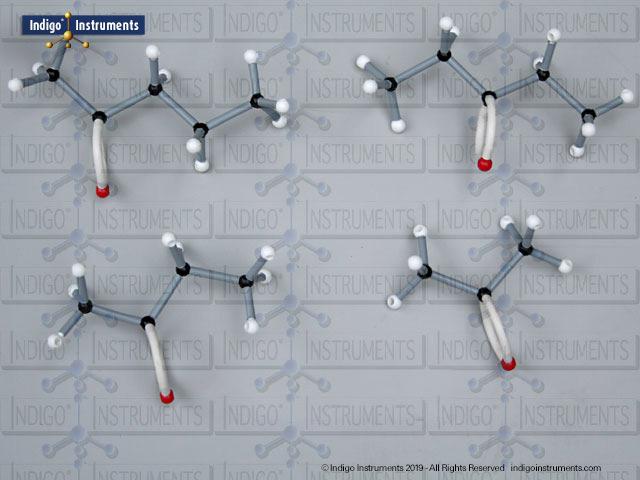
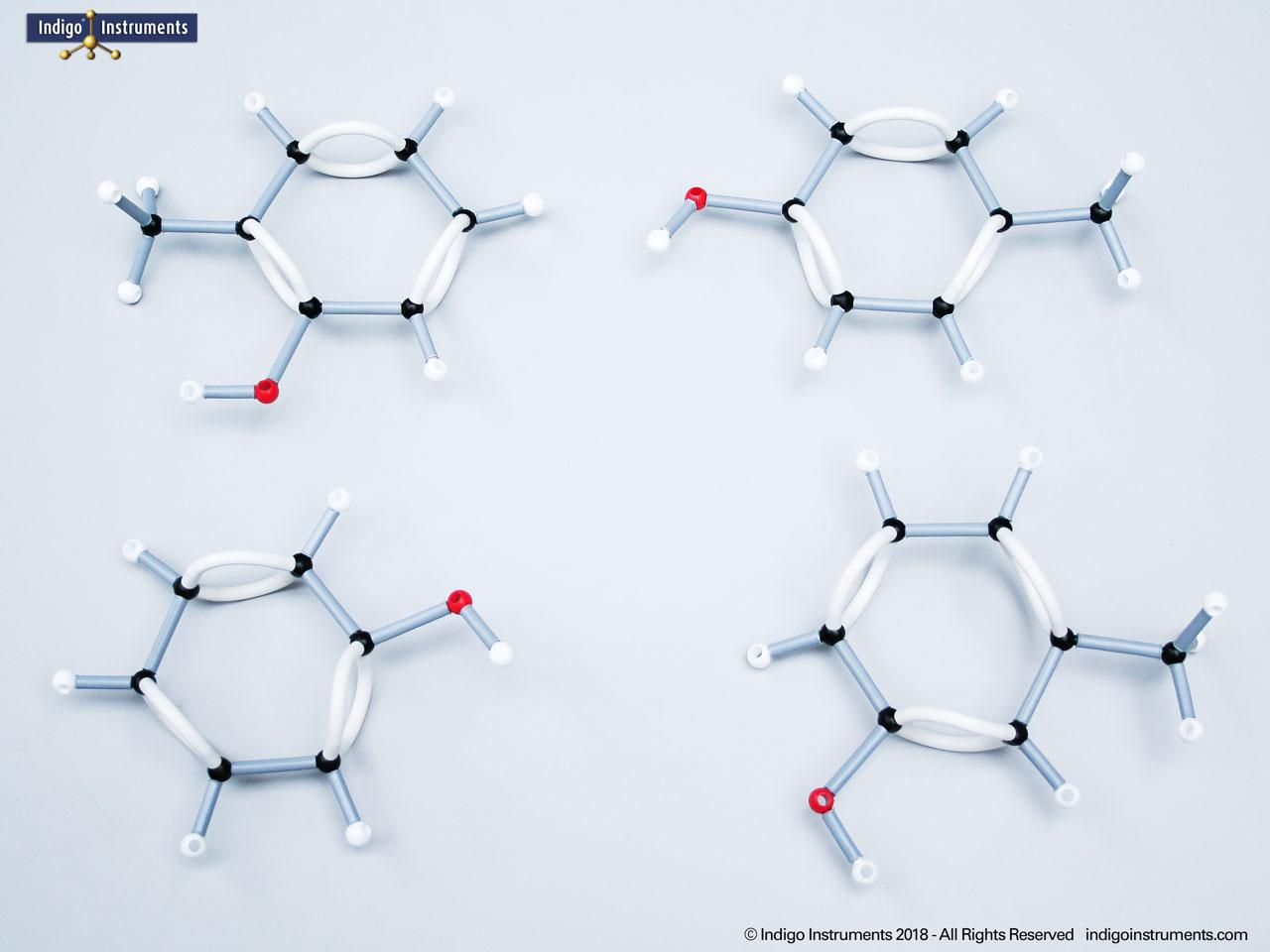
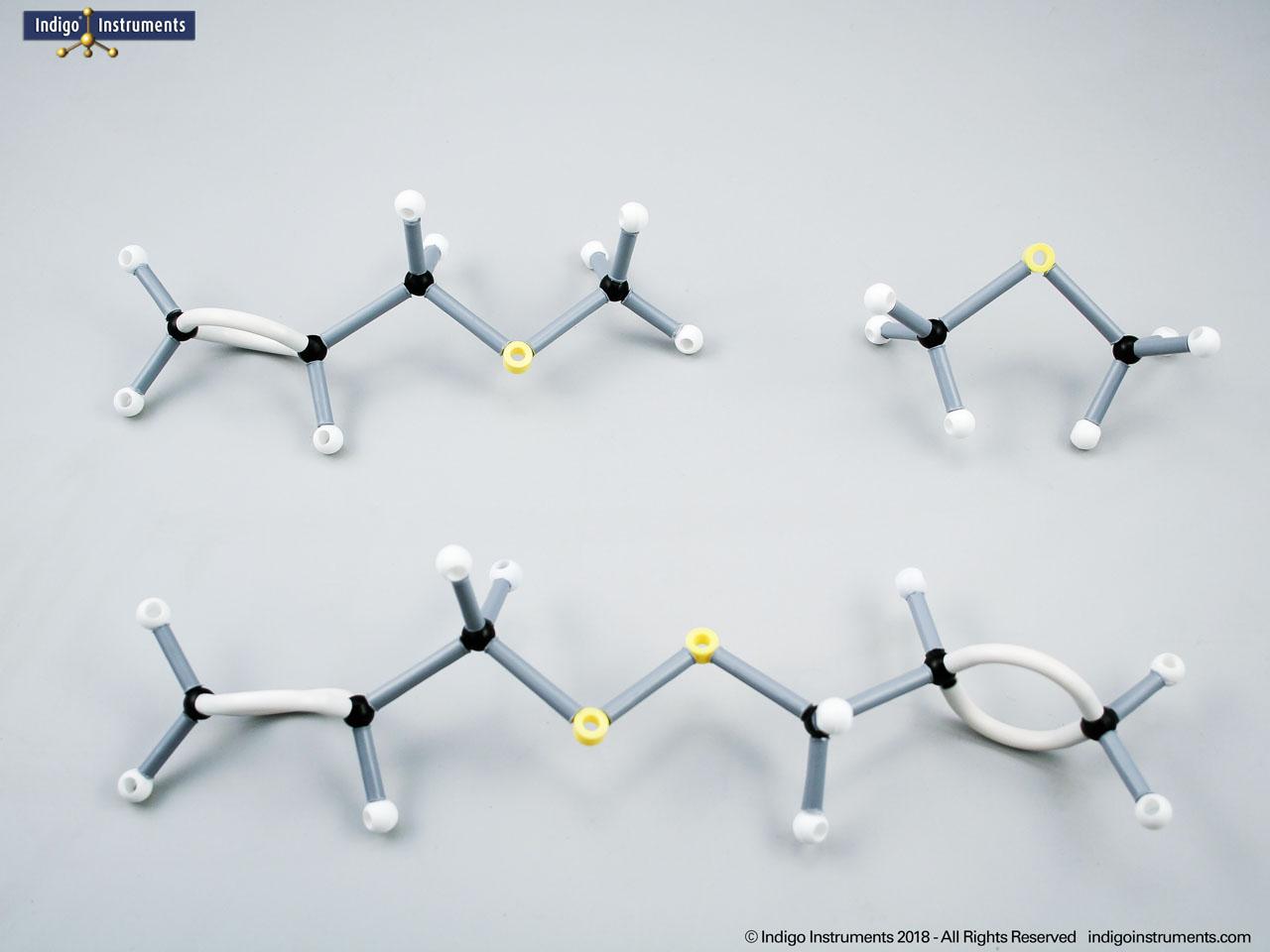
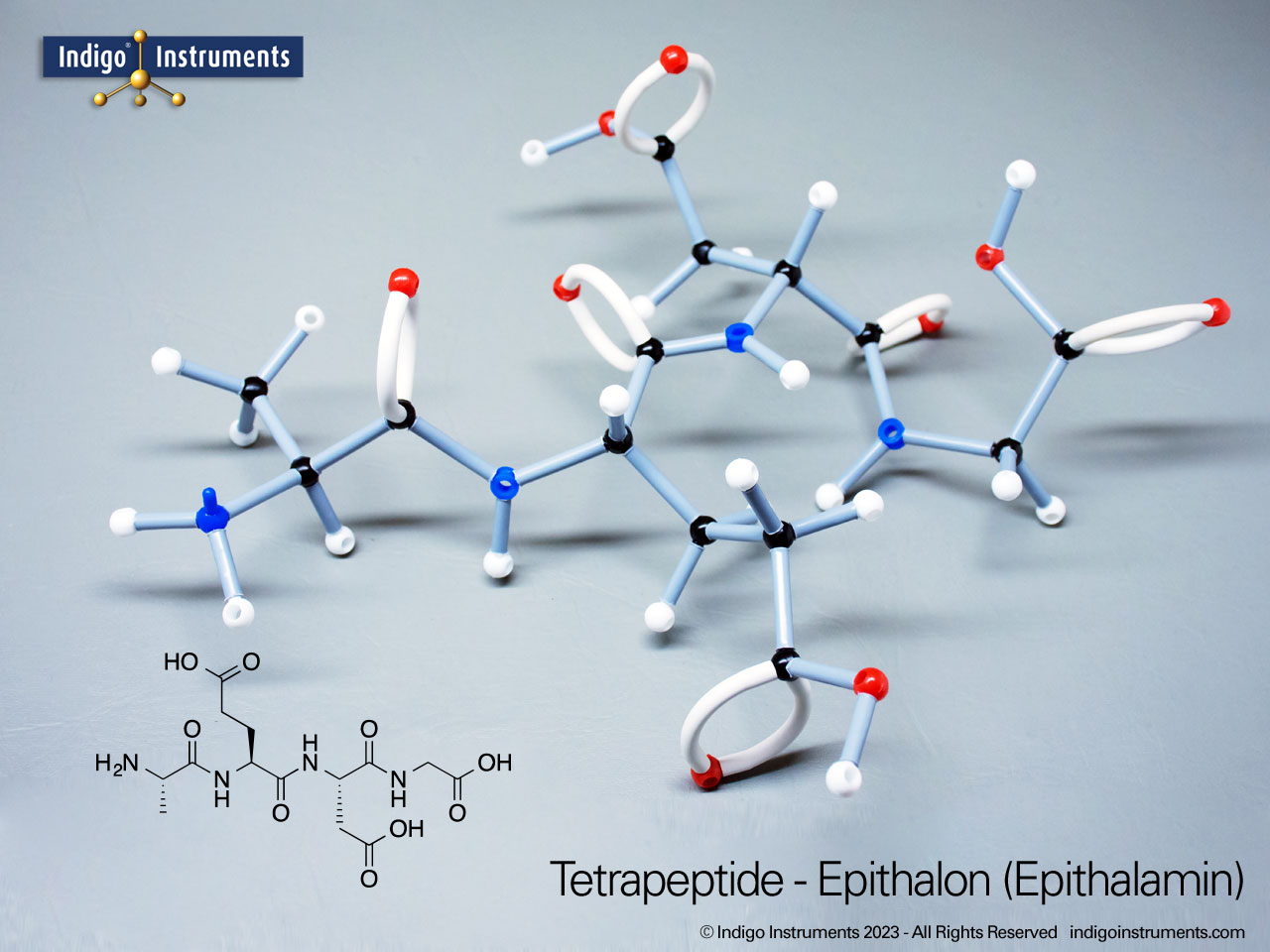
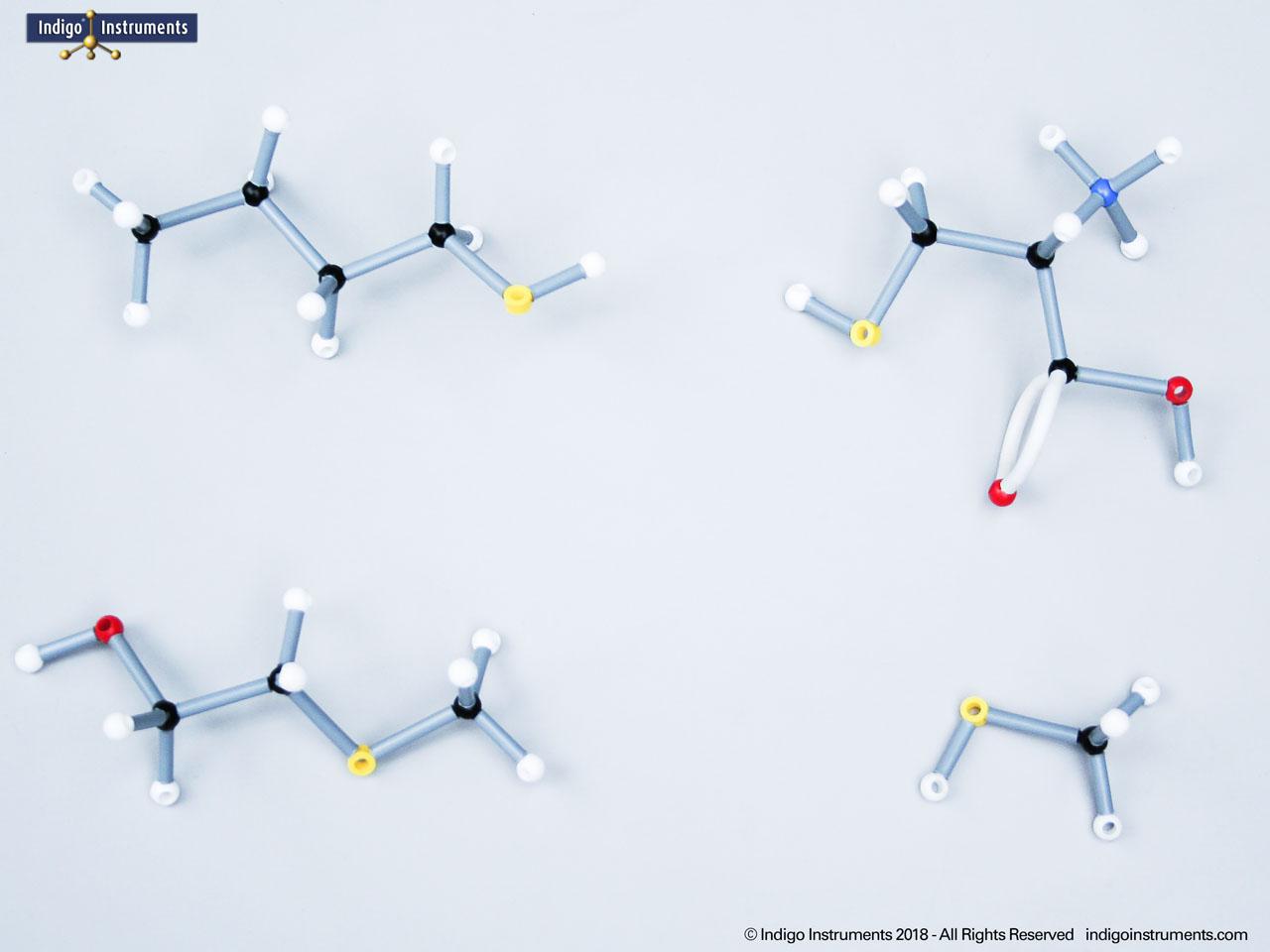
Alcohols are organic molecules containing a hydroxyl group (-OH) bonded to a carbon atom. In chemistry, they are key intermediates in synthesis and solvents in reactions. In biology, they occur naturally in sugars, amino acids, and lipids, and play roles in metabolism and signalling.
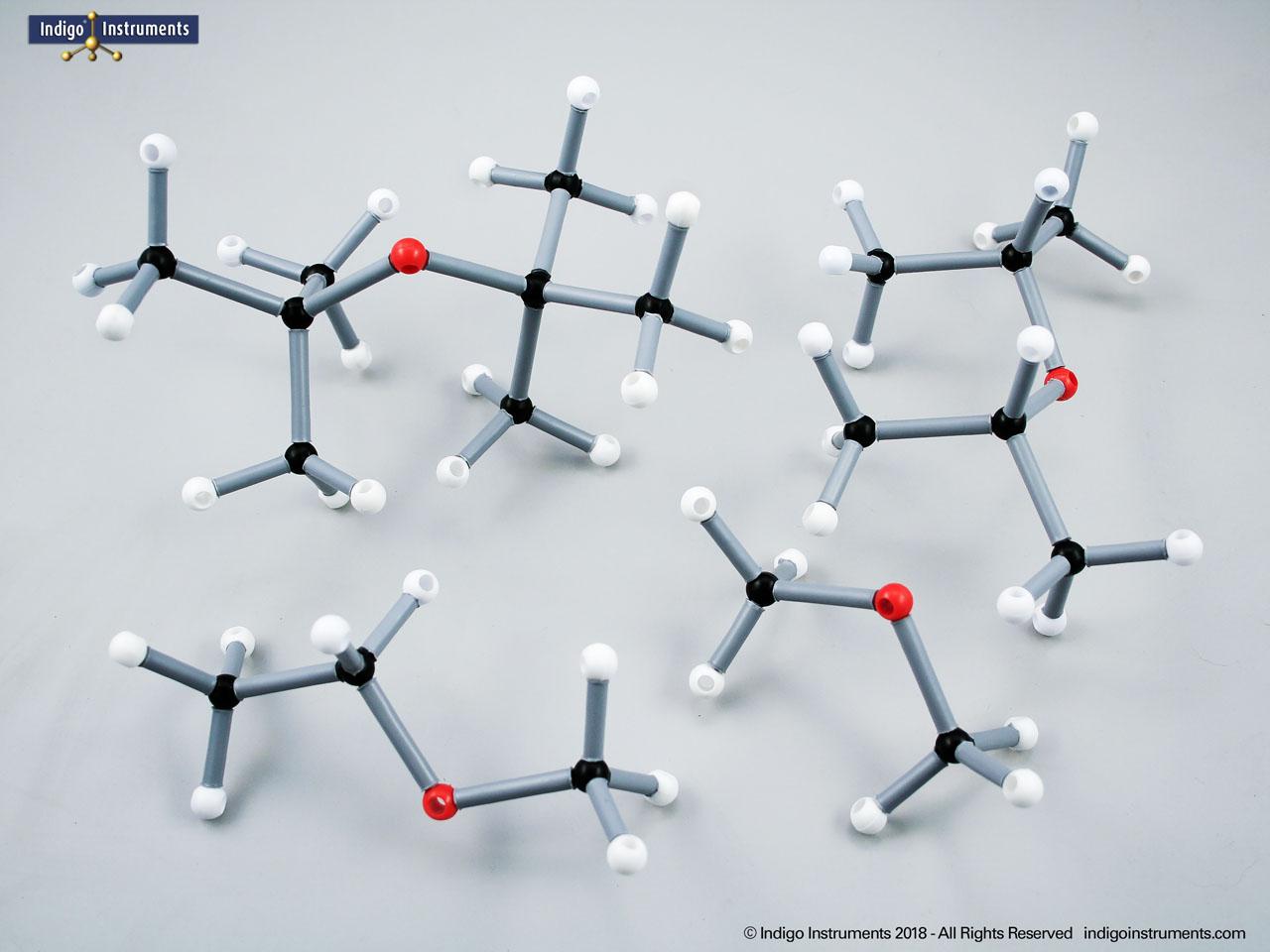
The structural formula of the hydroxy functional group, in ethanol for example, is C2H5OH compared to its molecular formula C2H6HO. The 68845NV Foundation model set includes six red 2 prong 110 degree oxygen atoms for depicting the OH hydroxyl. When combined with the set's 18 hydrogens & 16 sp3 carbons, you can build hundreds of alcohol structures to help learn IUPAC alcohol naming conventions as they relate to their parent alkanes.
Indigo Instruments has held inventory of genuine Cochranes of Oxford (Orbit) atoms & bonds for 30+ years. These parts are compatible with every molecular model set we have sold since day 1. This quality may appear expensive but no parts support from other vendors costs even more.