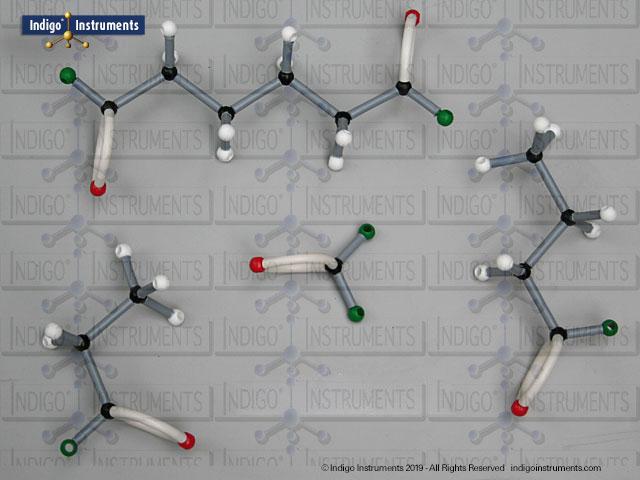
Glycylglycine Planar Peptide Bond-MCAT Study
SKU: 68845NV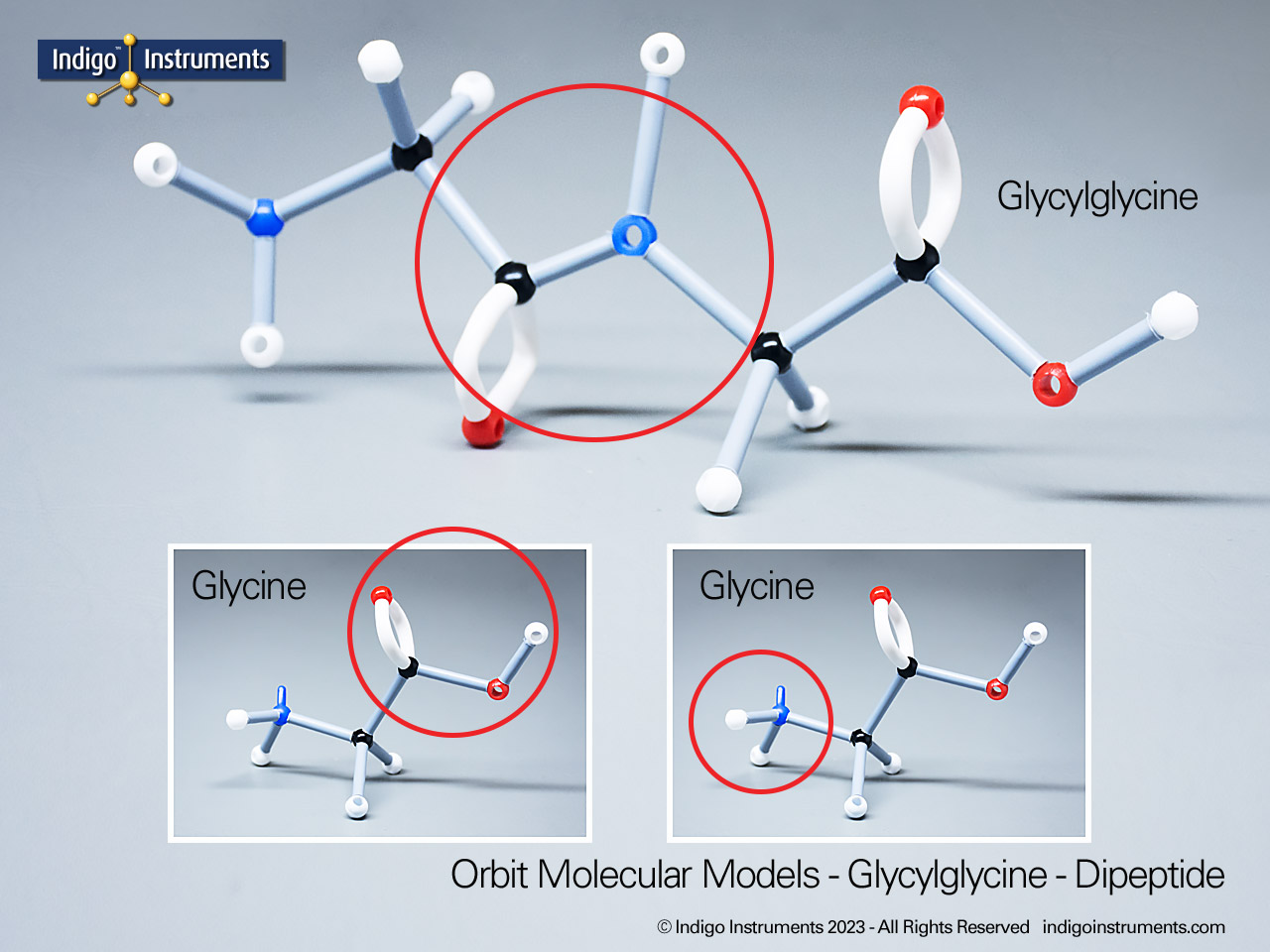
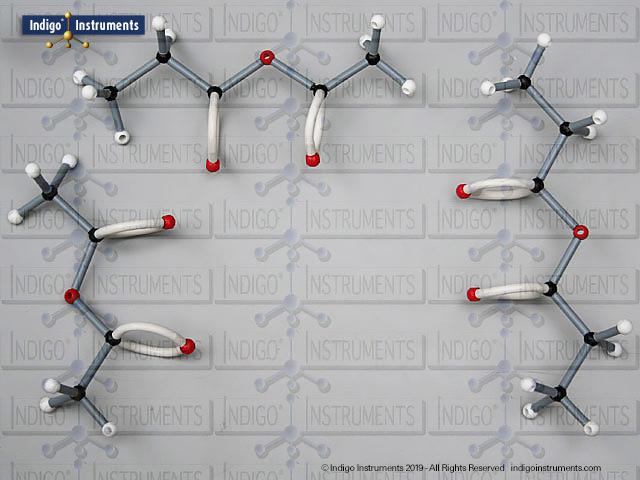
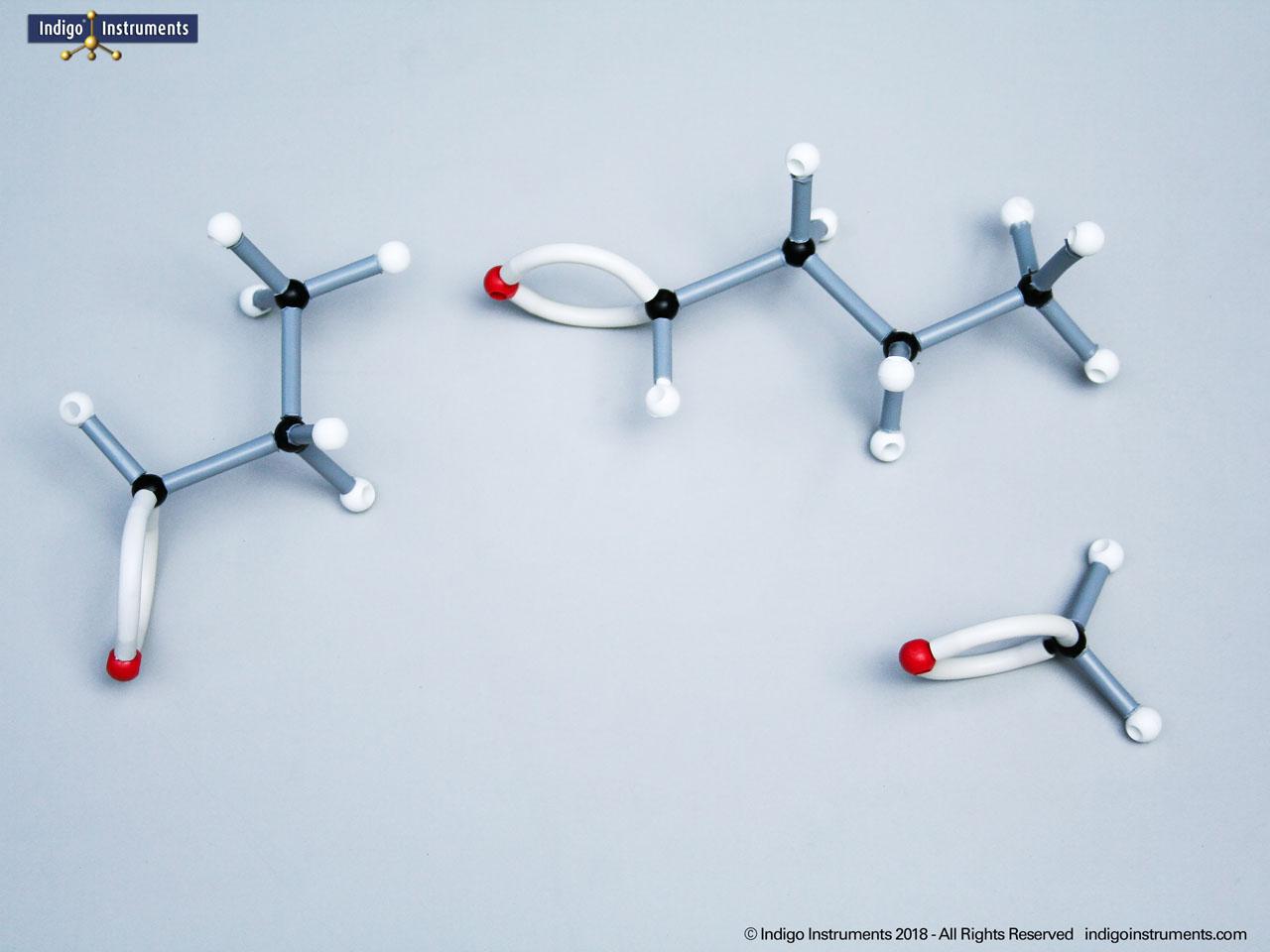
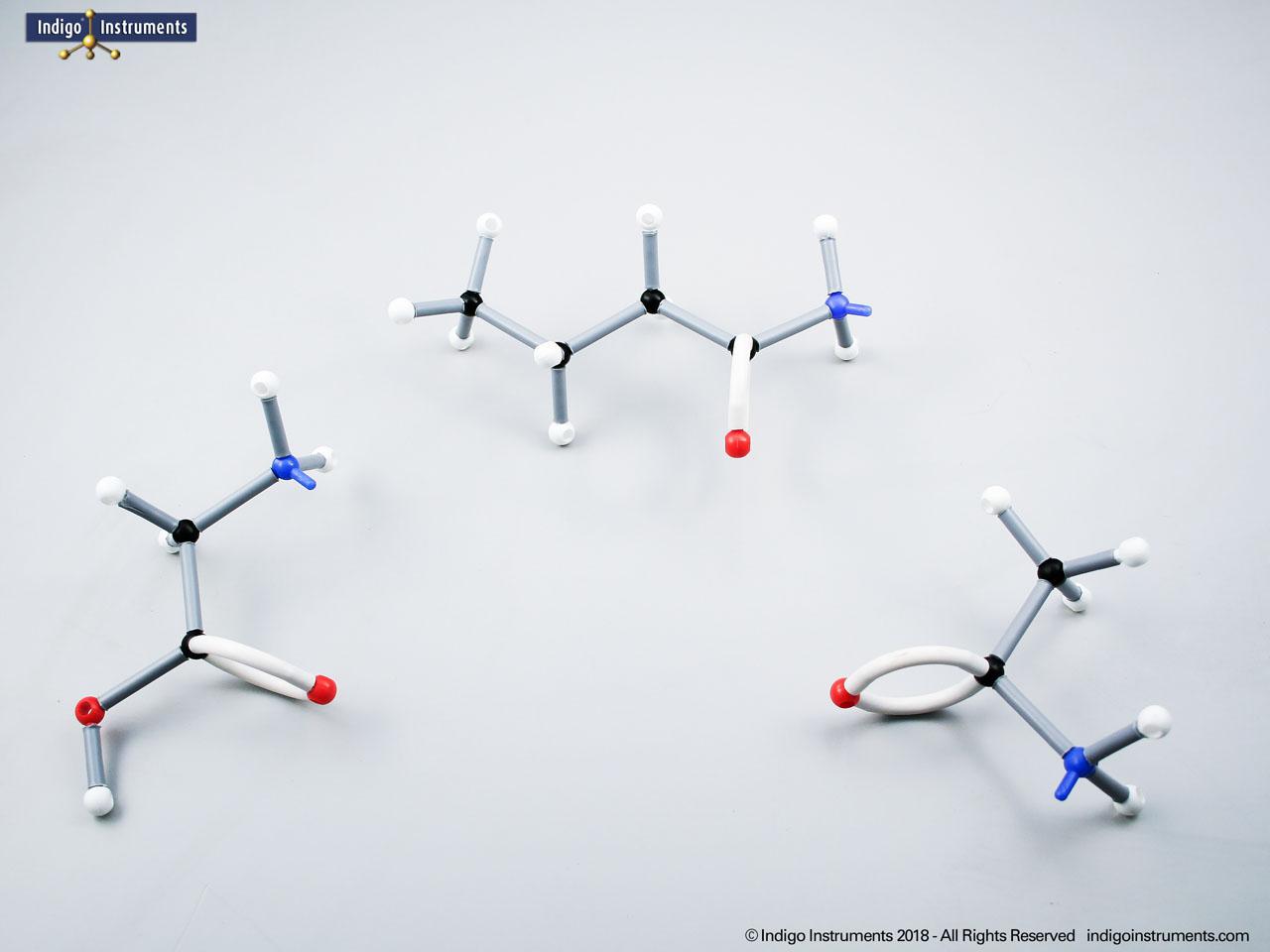
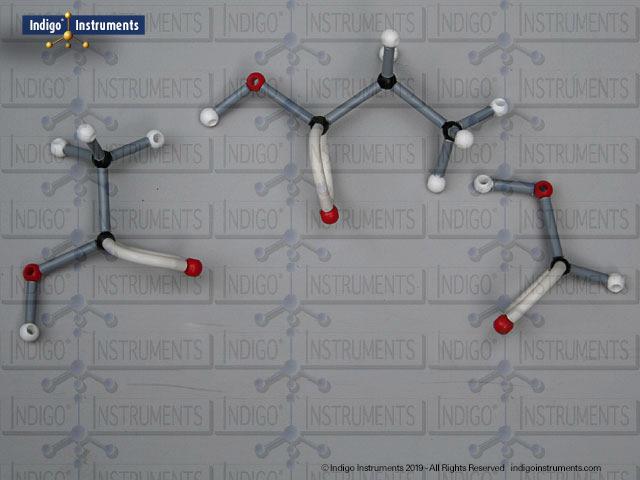
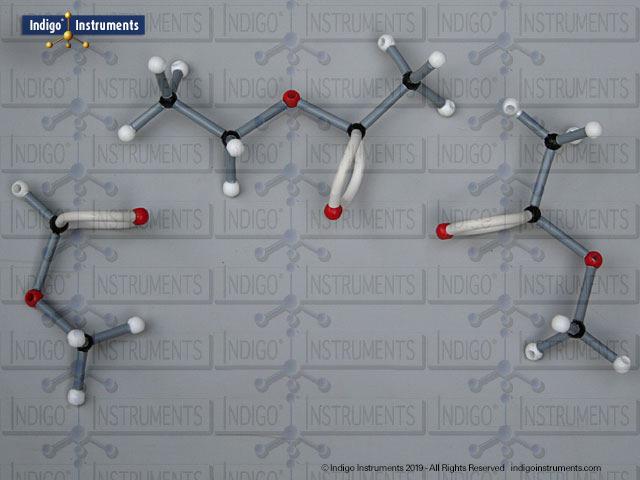
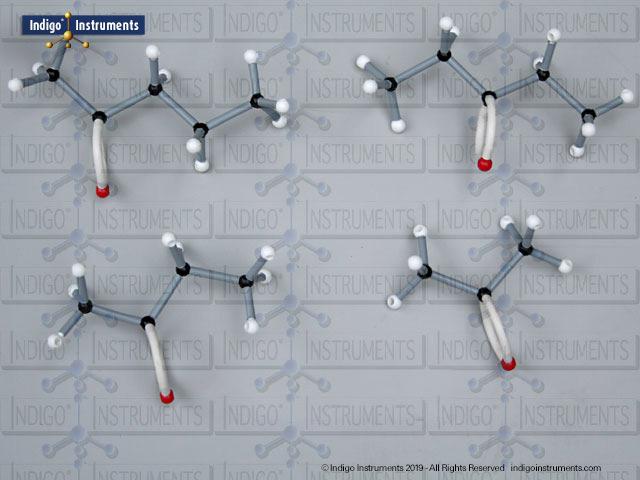
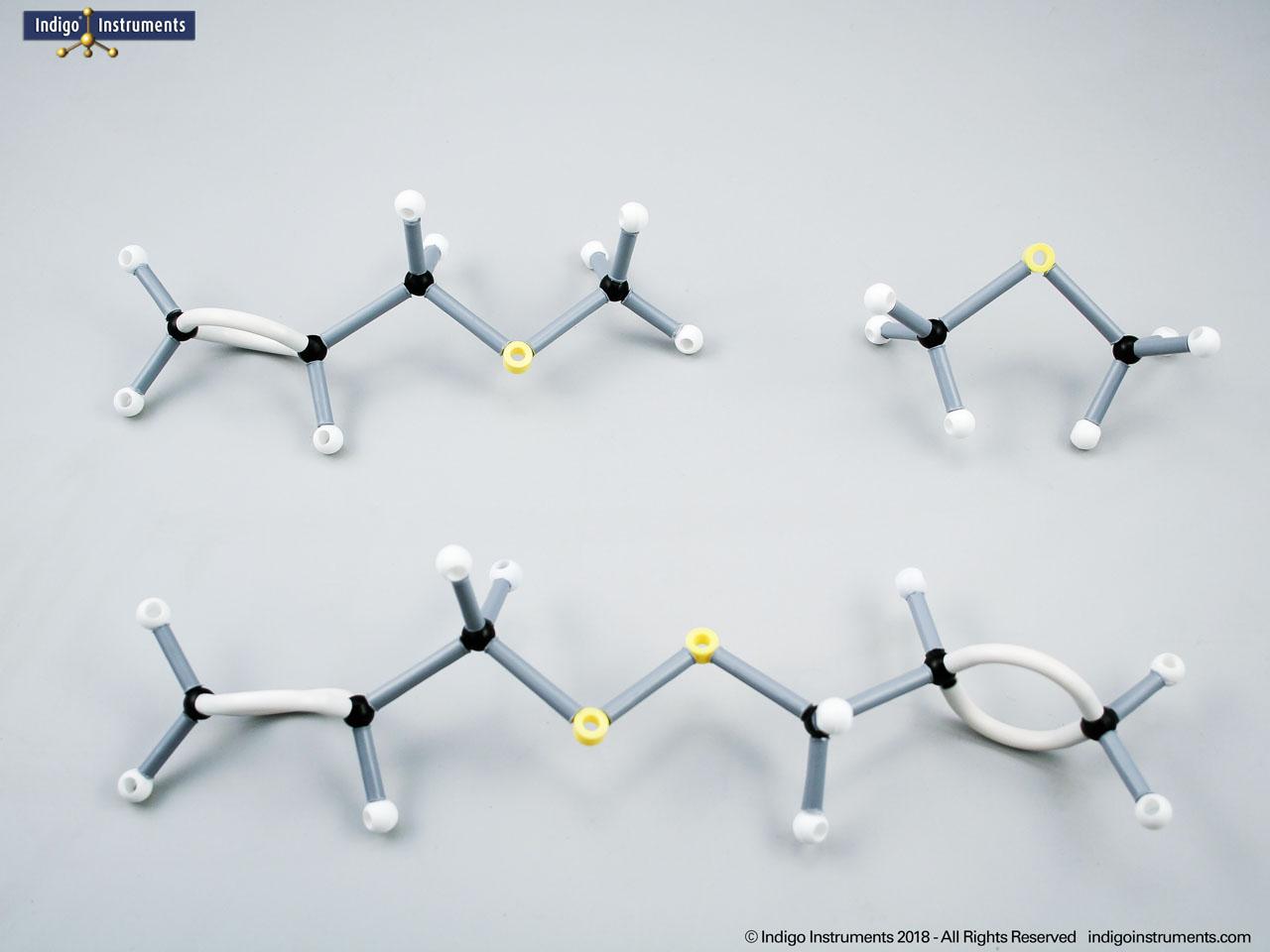
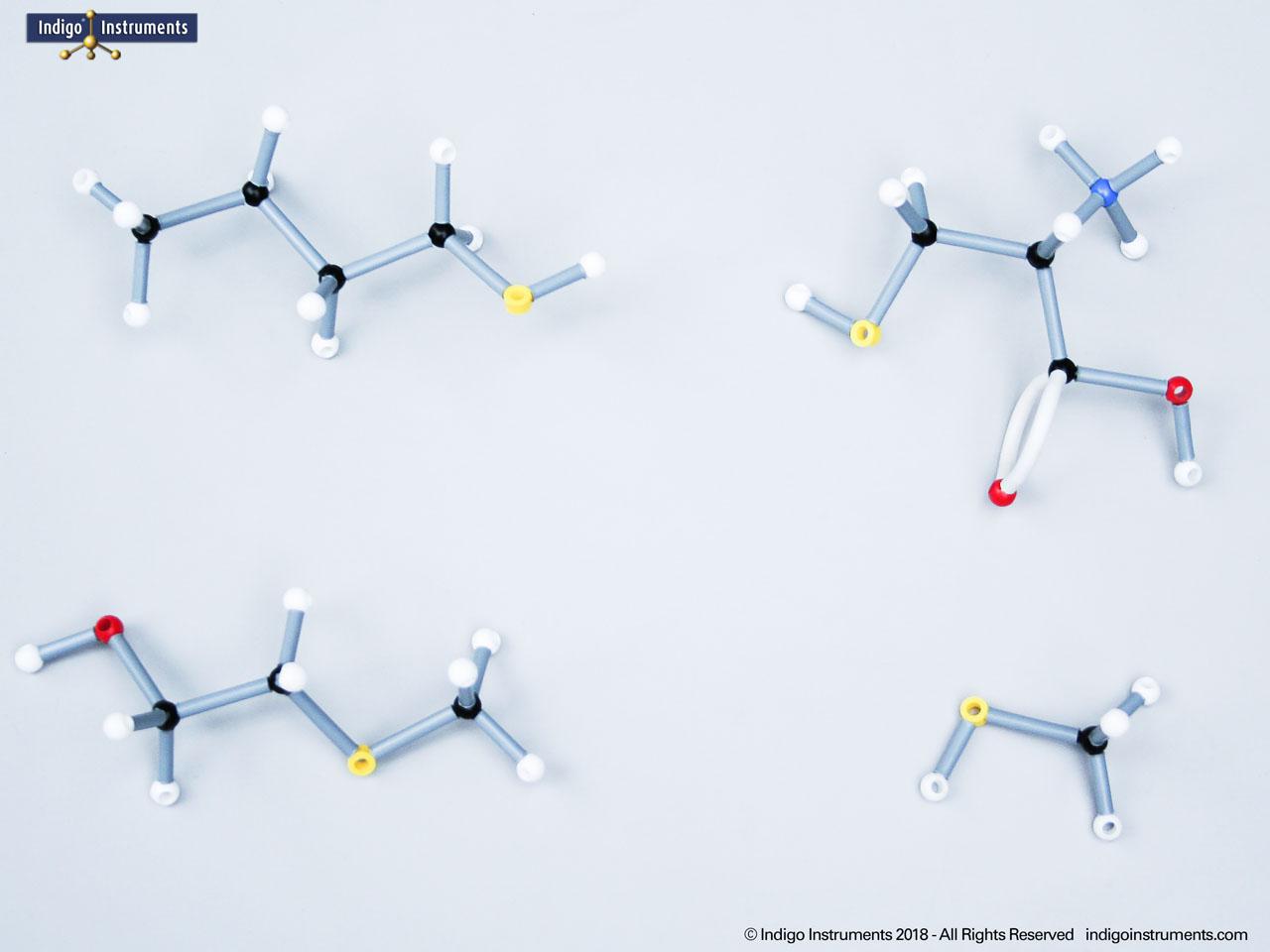
Building amino acid models is one an effective way to visualize peptide bond formation and understand a fundamental characteristic of protein structure. Our model of glycylglycine, a dipeptide made of two glycine units, is a good introduction to how amino acids link via condensation reactions to form peptides and proteins.
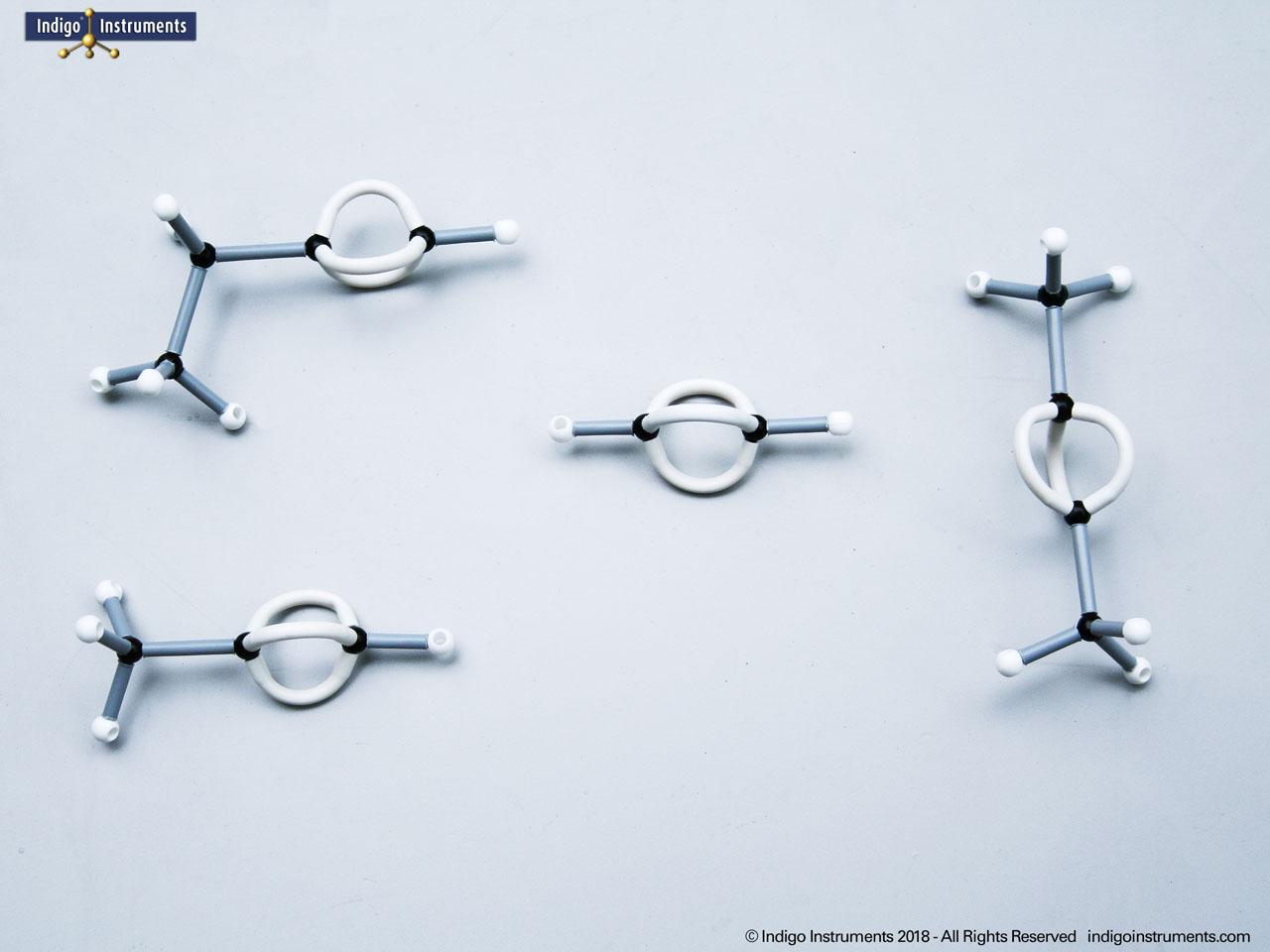
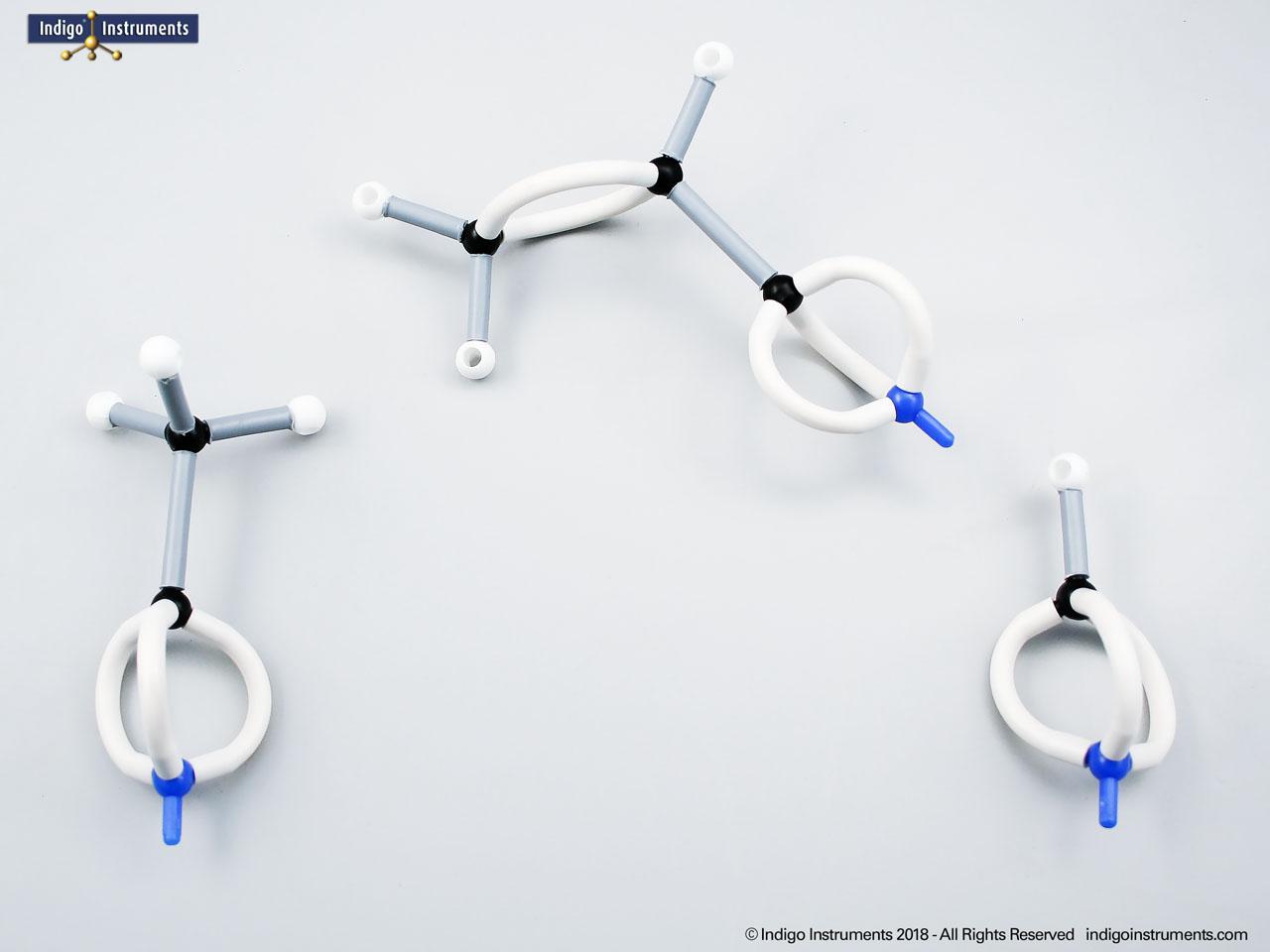
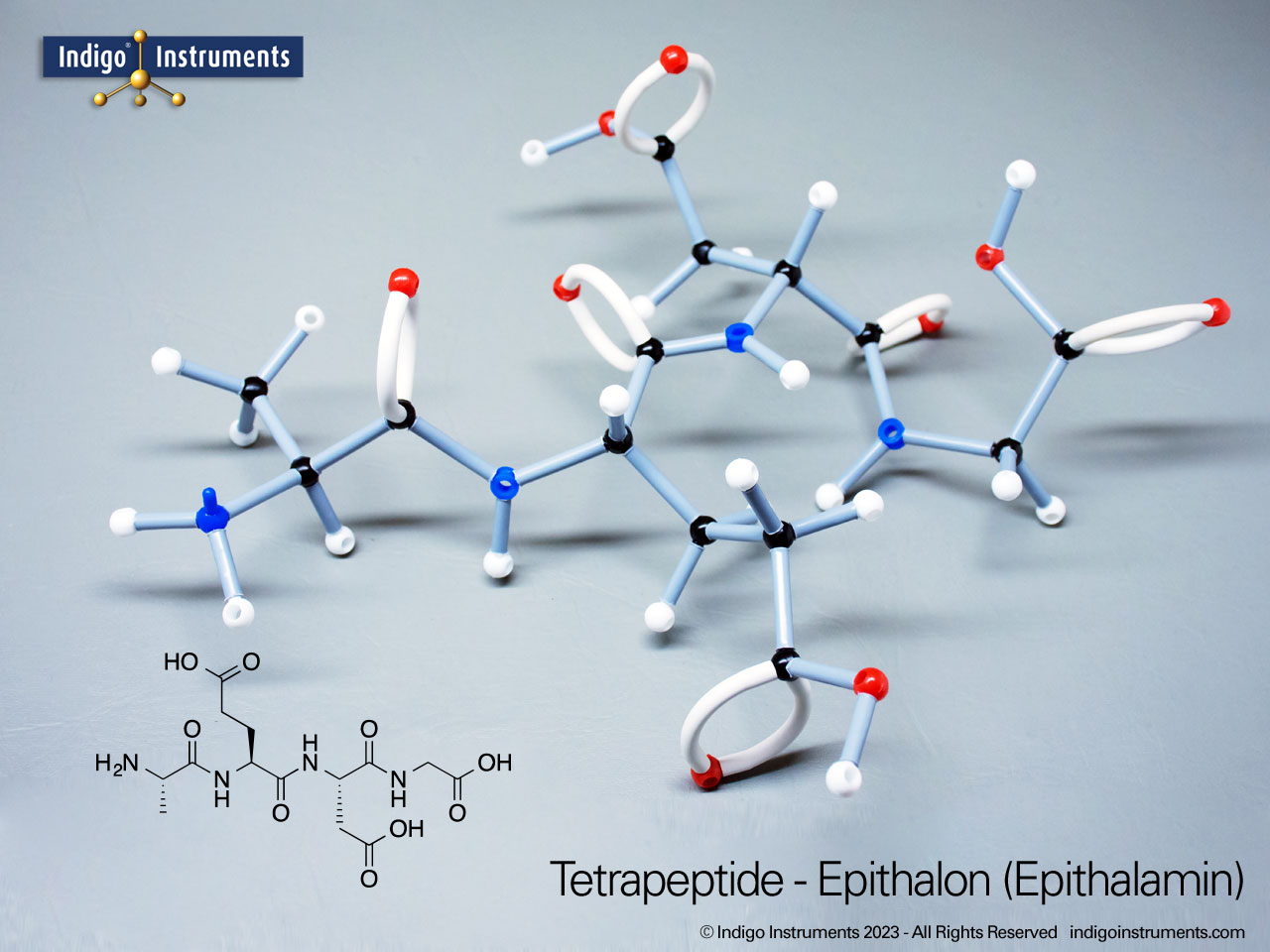
Aspartame, for example, is a well-known artificial sweetener that highlights real-world relevance: it is a dipeptide composed of the amino acids phenylalanine (Phe) and aspartic acid (Asp), with the molecular formula C??H??N?O?. By modelling such dipeptides, students can clearly observe how peptide bonds form and how the geometry of the nitrogen atom changes—from tetrahedral in free amines to planar when involved in a peptide bond.
These changes are not just structural—they’re key to understanding enzyme activity, protein folding, and the chemical properties that drive biological function. Whether you're studying for a biochemistry midterm or reviewing amino acid structures for the MCAT exams, building these molecular models reinforces learning through tactile and visual feedback.
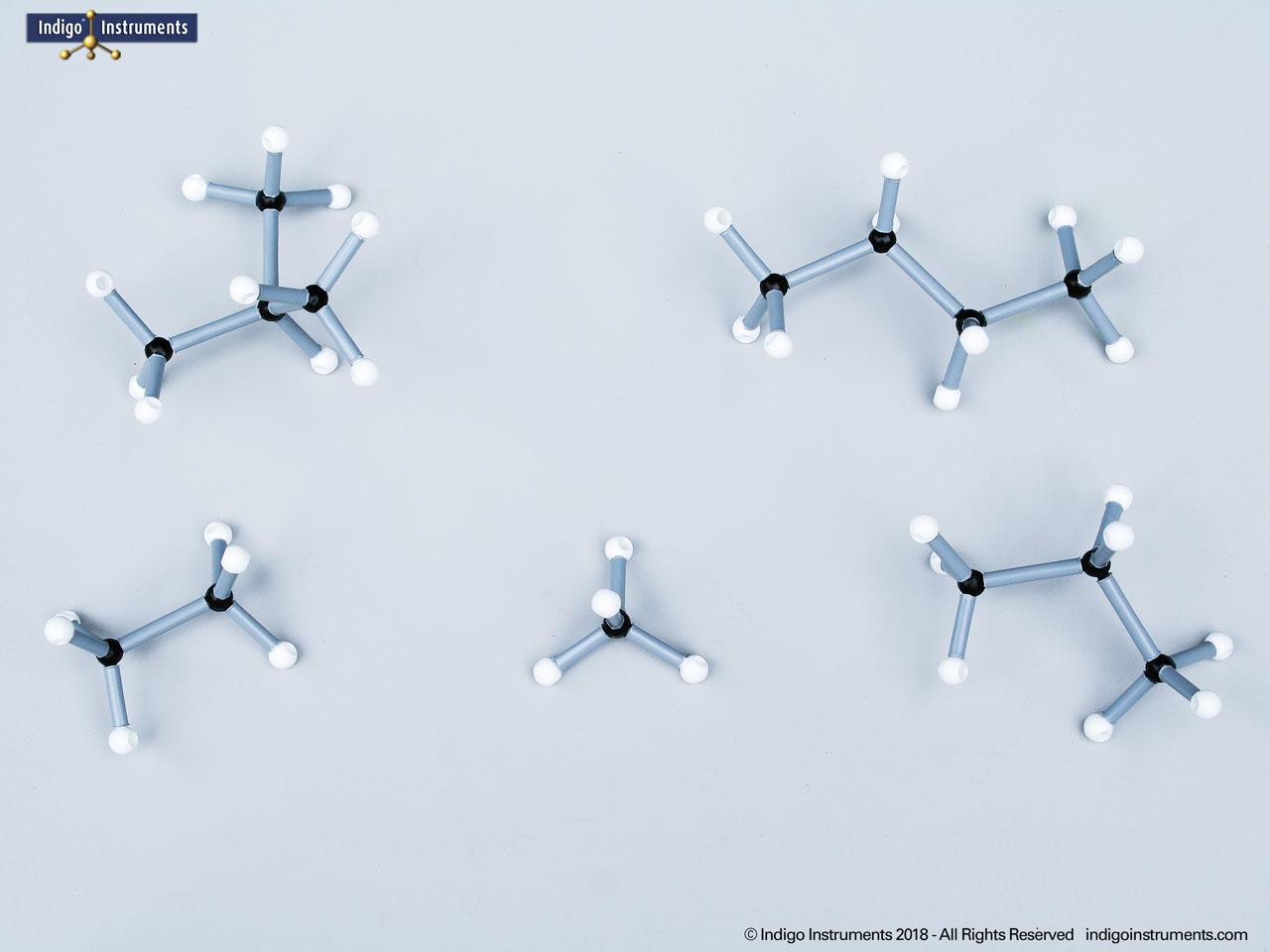
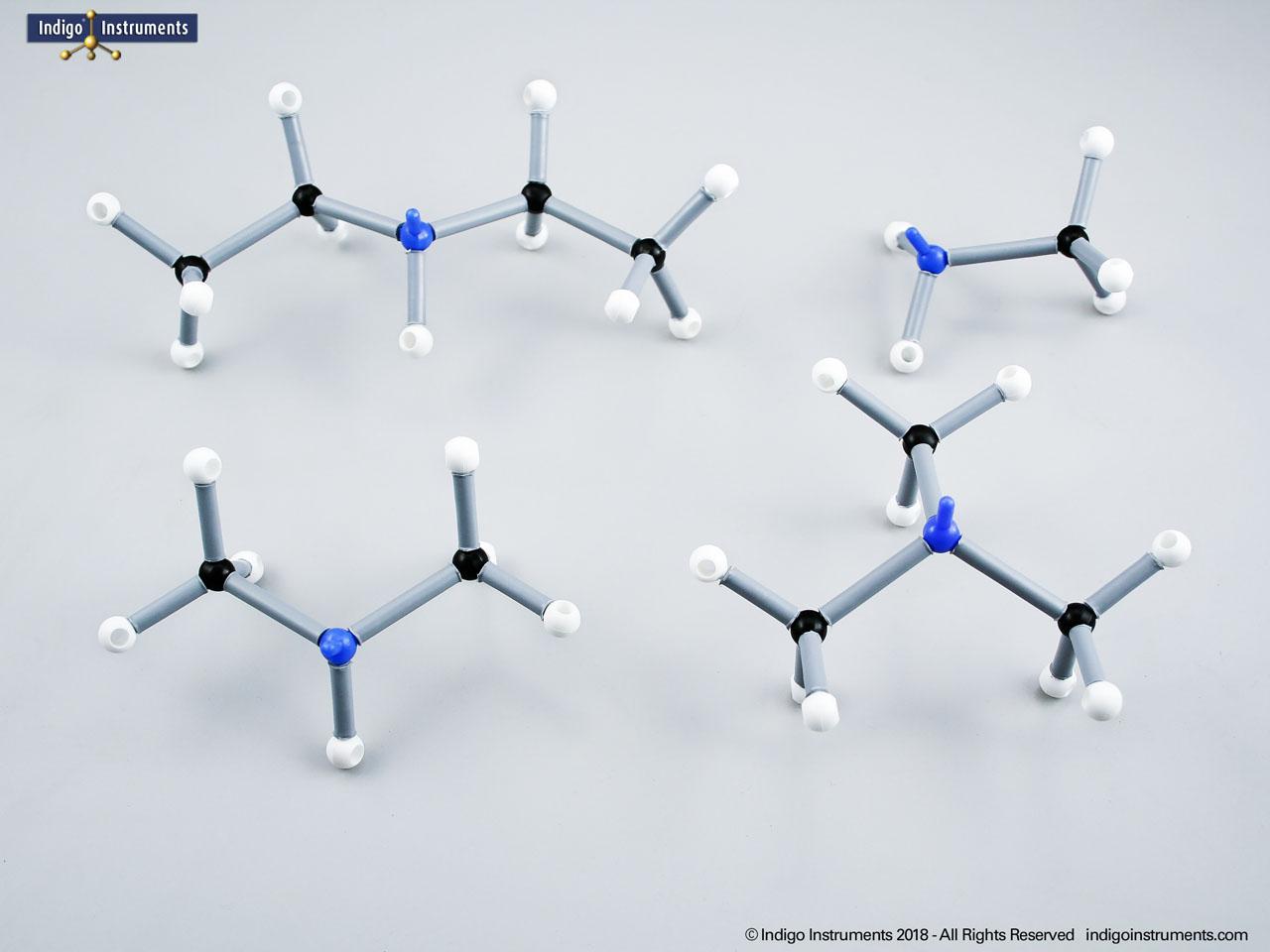
Note: This model set comes with 2 tetrahedral & 1 planar nitrogen. When combining two amino acids via a peptide bond, the planar version is substituted for one of the tetrahedra as shown in the image.
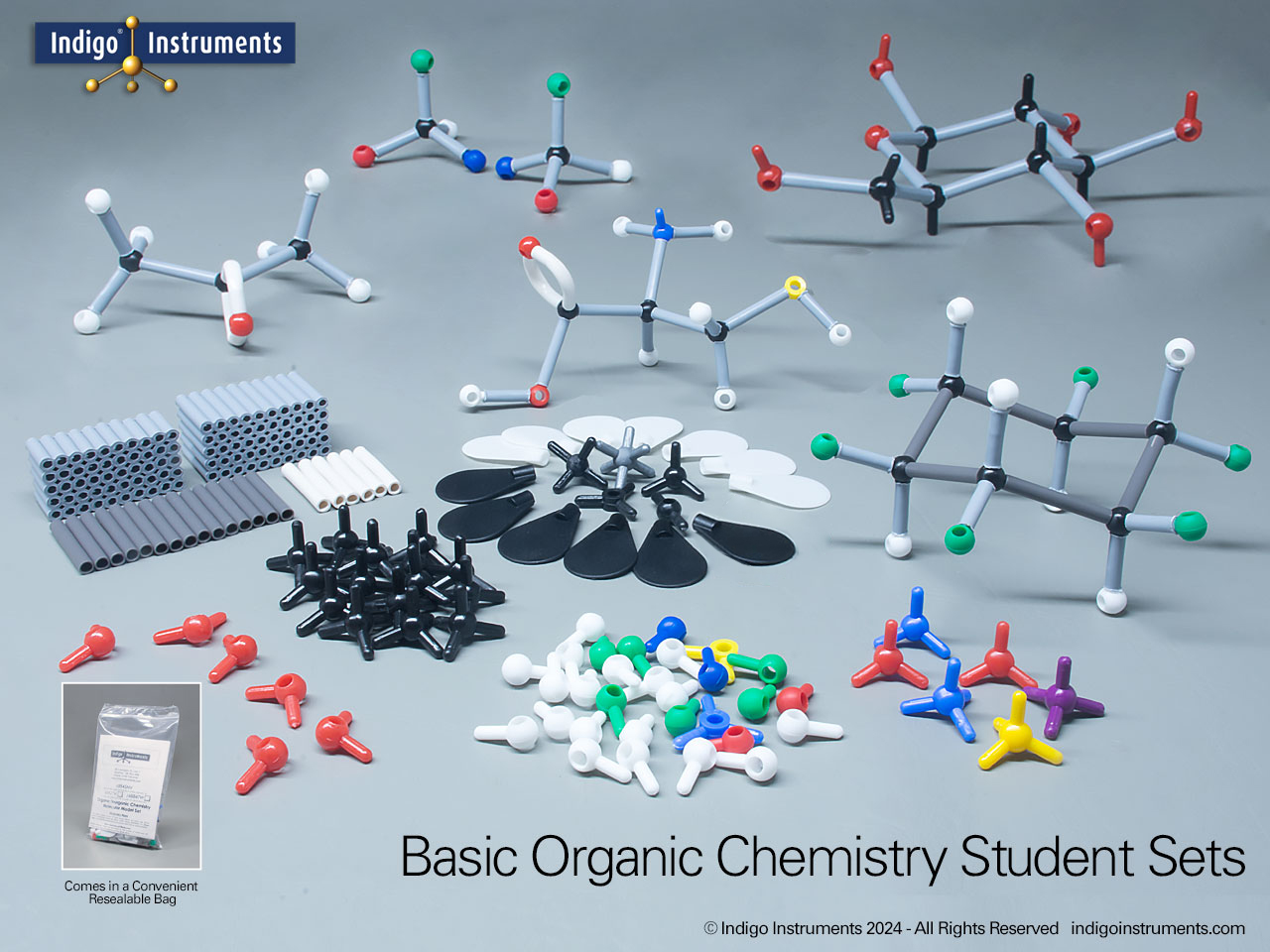
Indigo Instruments has maintained a substantial inventory of genuine Cochranes of Oxford (Orbit) atoms & bonds for over 30+ years (scroll down to see "Skeletal (Orbit/Minit). These parts are compatible with every molecular model set we have sold since day 1. This level of quality may appear expensive but no parts support from other vendors costs even more.