Trigonal Bipyramidal Geometry Molecular Model
SKU: 68823W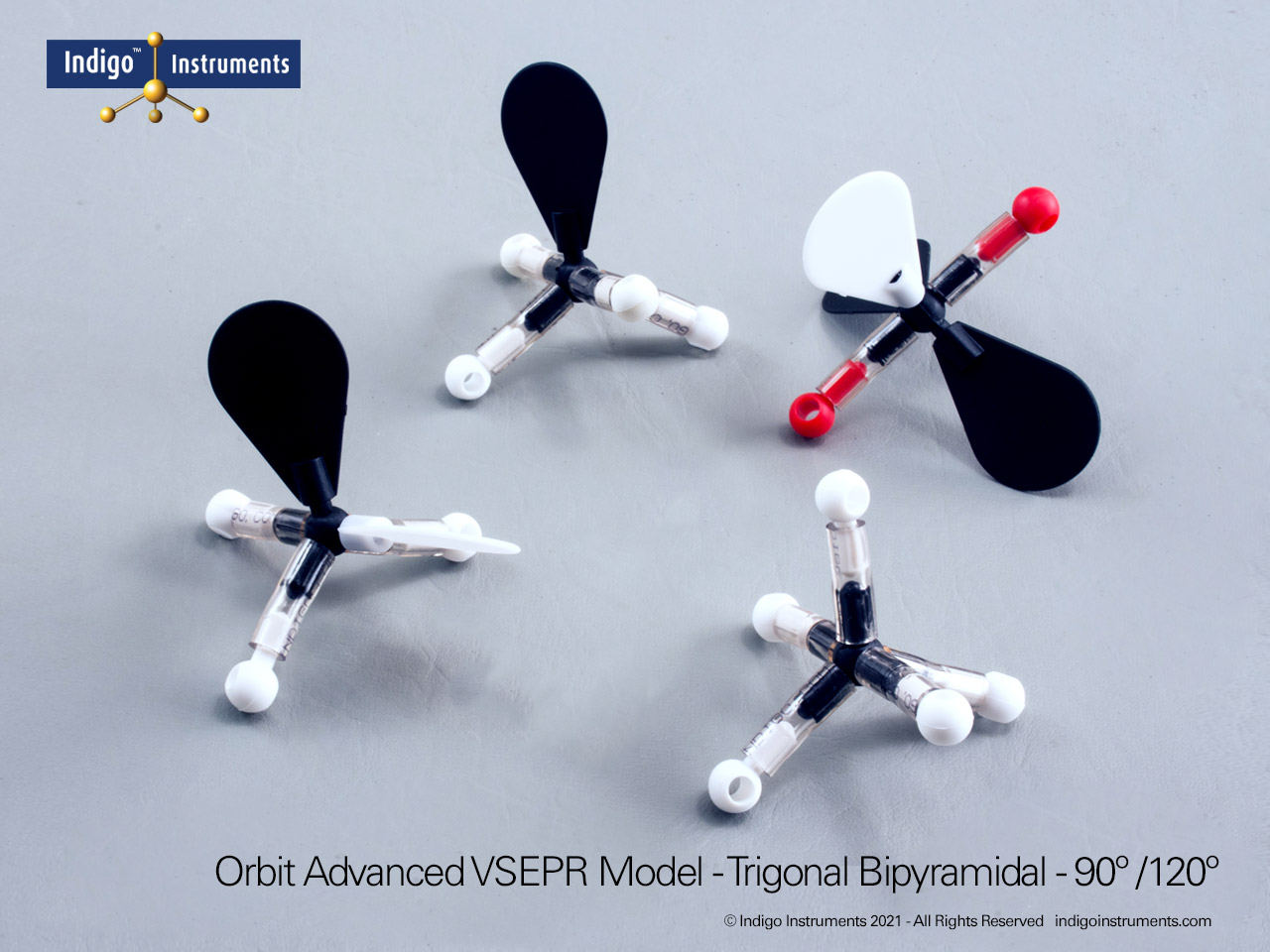
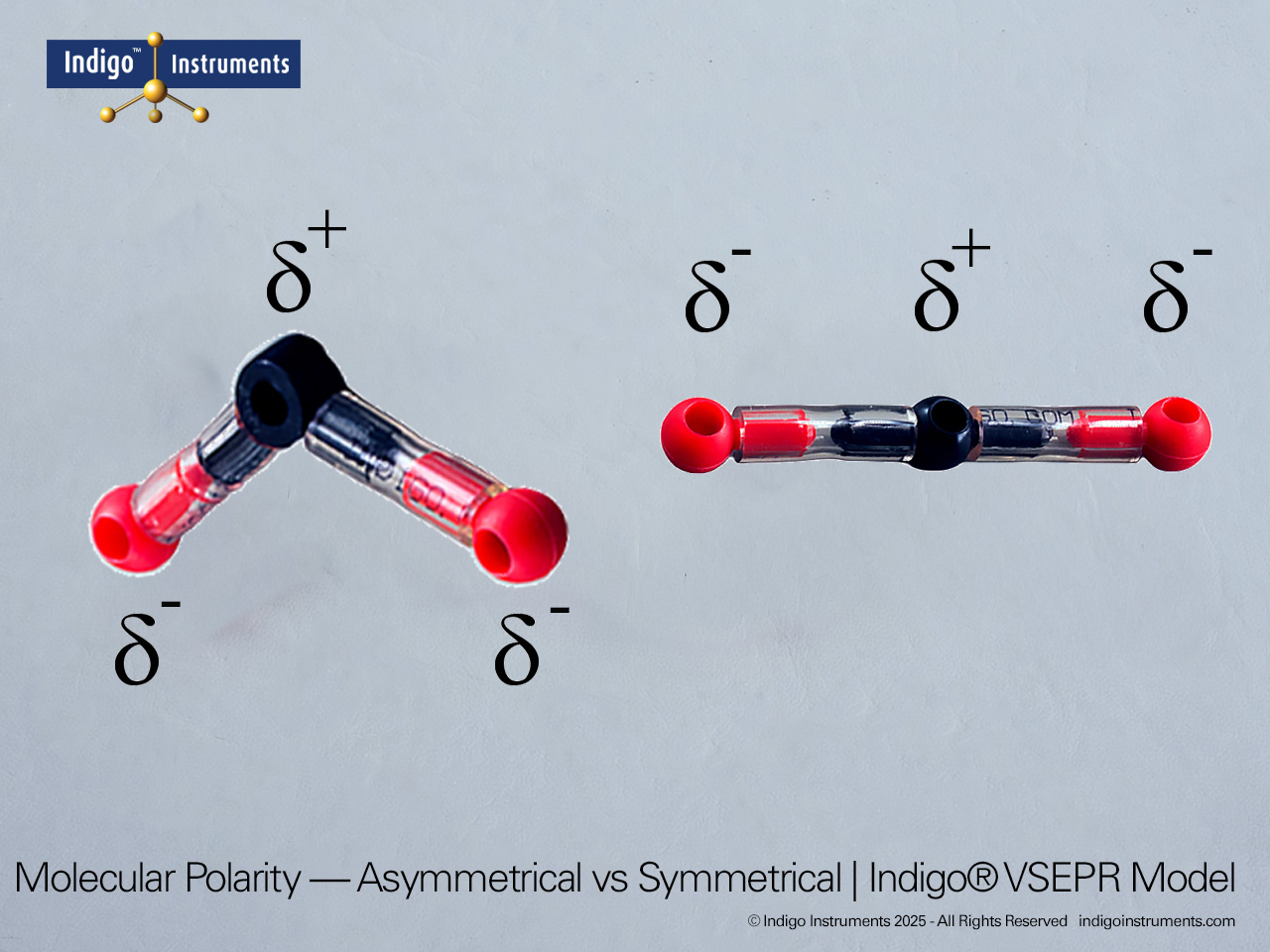
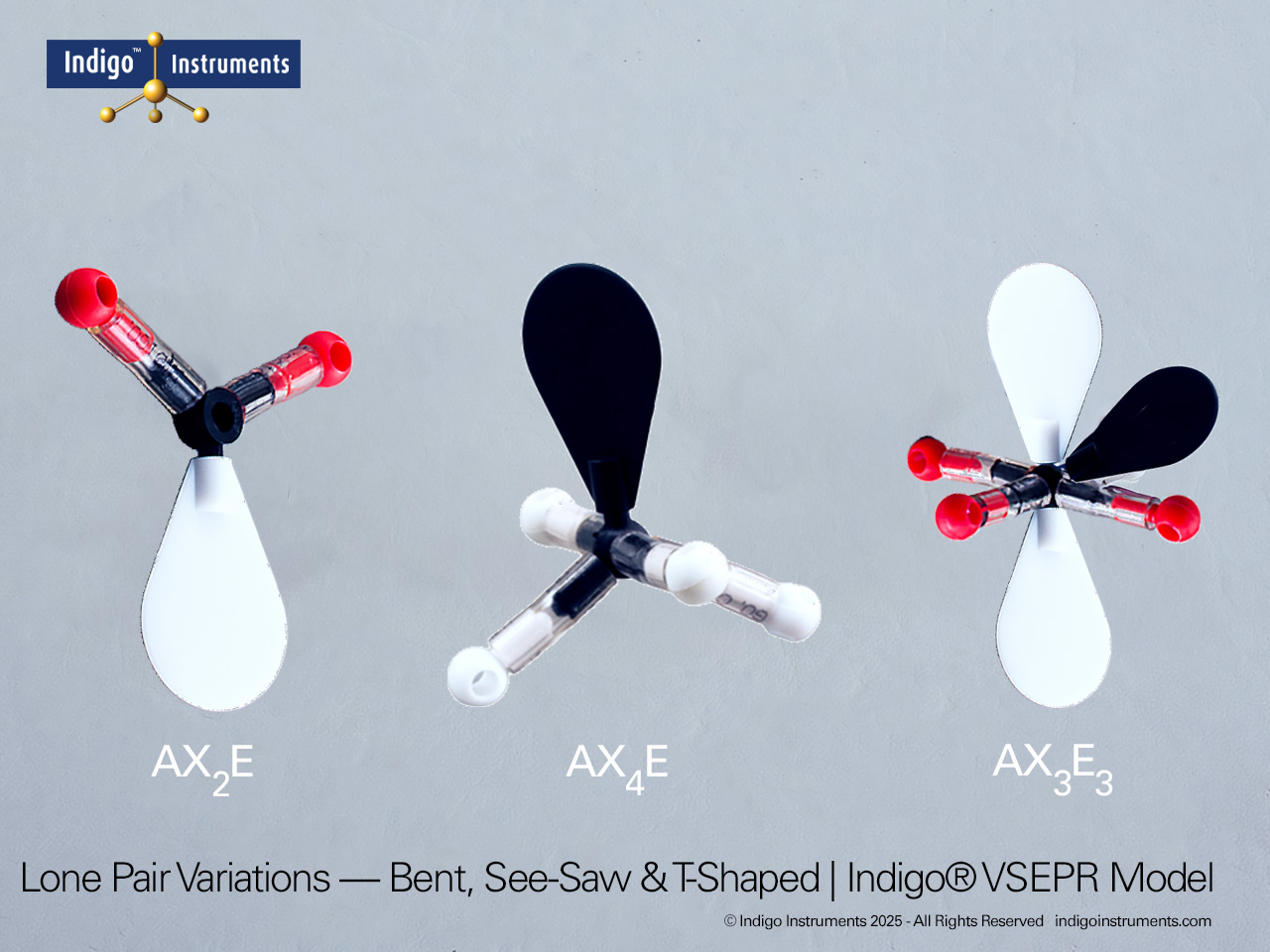
Explore trigonal bipyramidal molecular geometry using the Indigo® VSEPR theory model set. Build AX5 shapes like PF5 and PCl5 to visualize 90° and 120° bond angles.
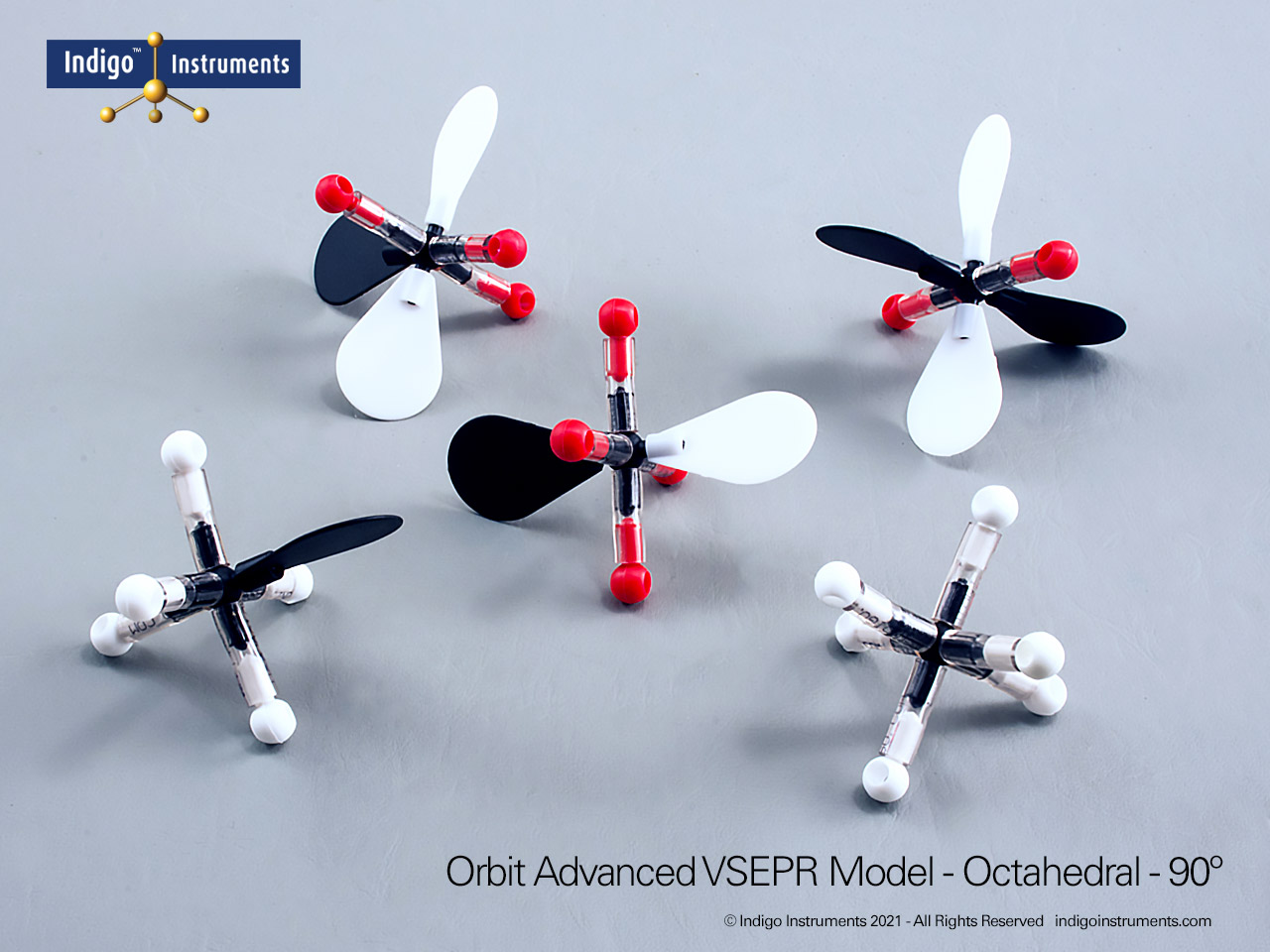
Visualize trigonal bipyramidal molecular geometry with the Indigo® VSEPR Model Kit. Build the AX5 structure to show three equatorial bonds 120° apart and two axial bonds 90° from the plane. This geometry demonstrates how five regions of electron density arrange to minimize repulsion, forming a stable three-dimensional shape.
The Lewis structure of this molecule has trigonal bipyramidal electron geometry which produces VSEPR model shapes with 3 120 degree bond angles that are at right angles to a 180 degree pair.