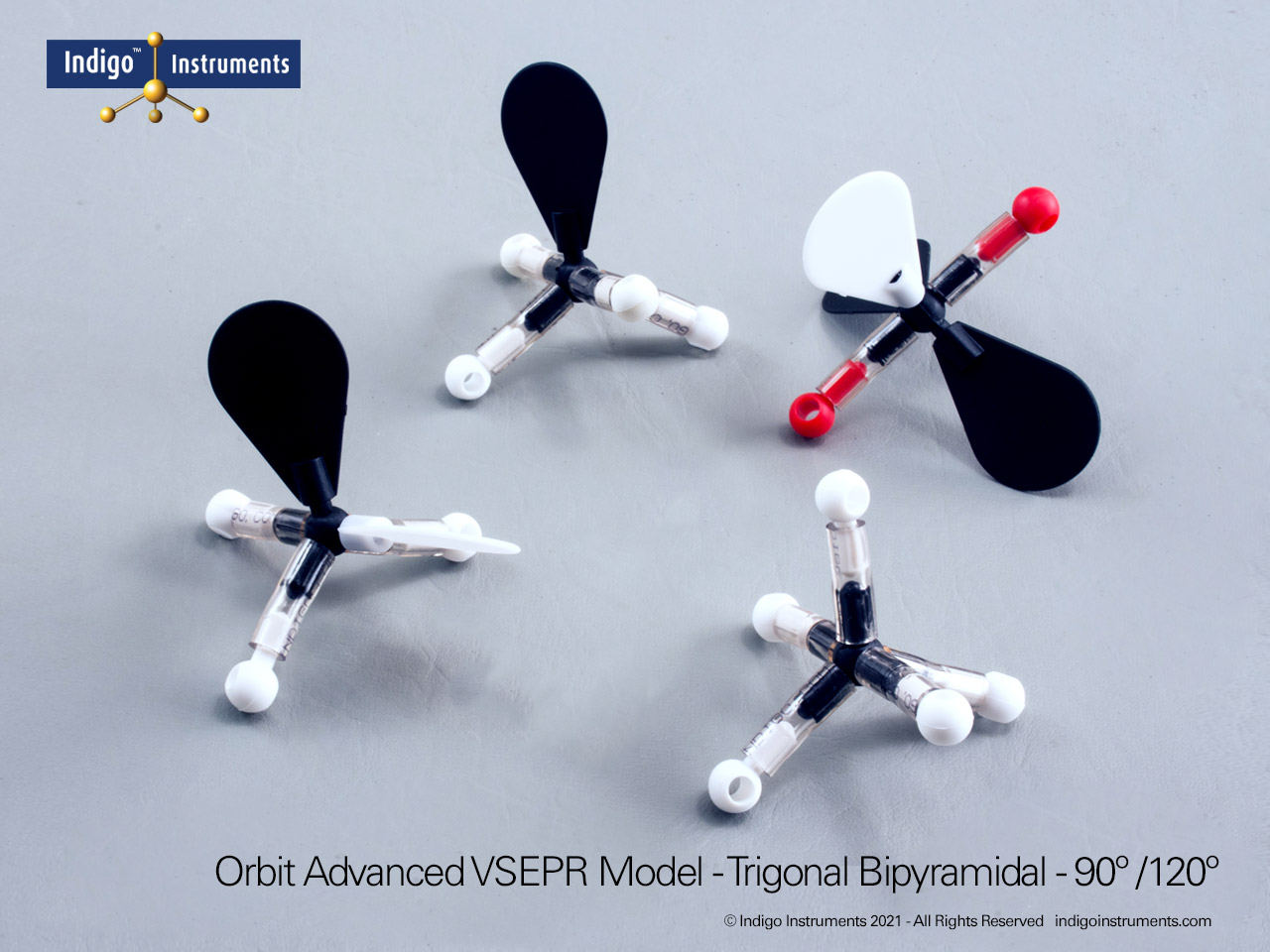
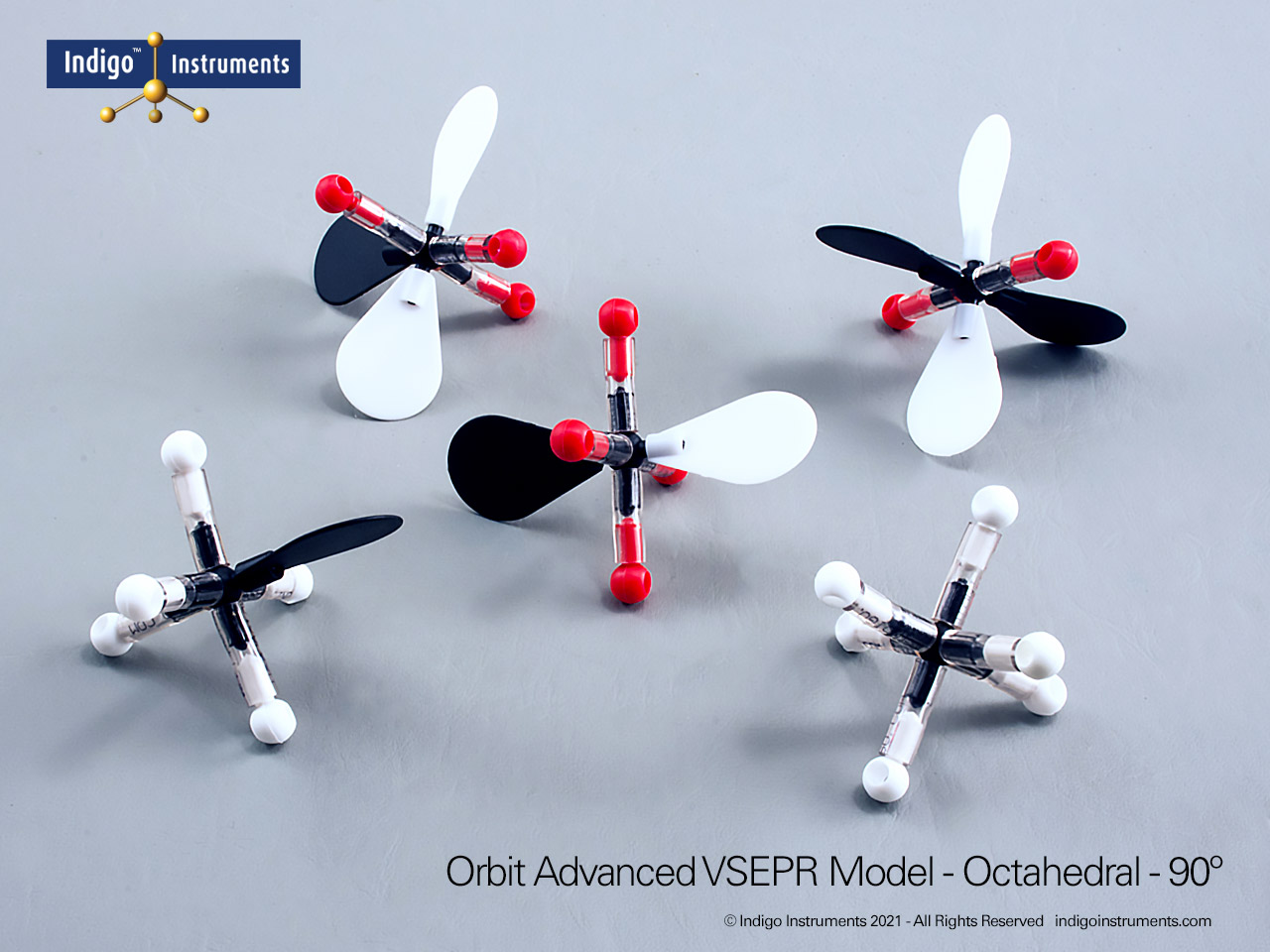
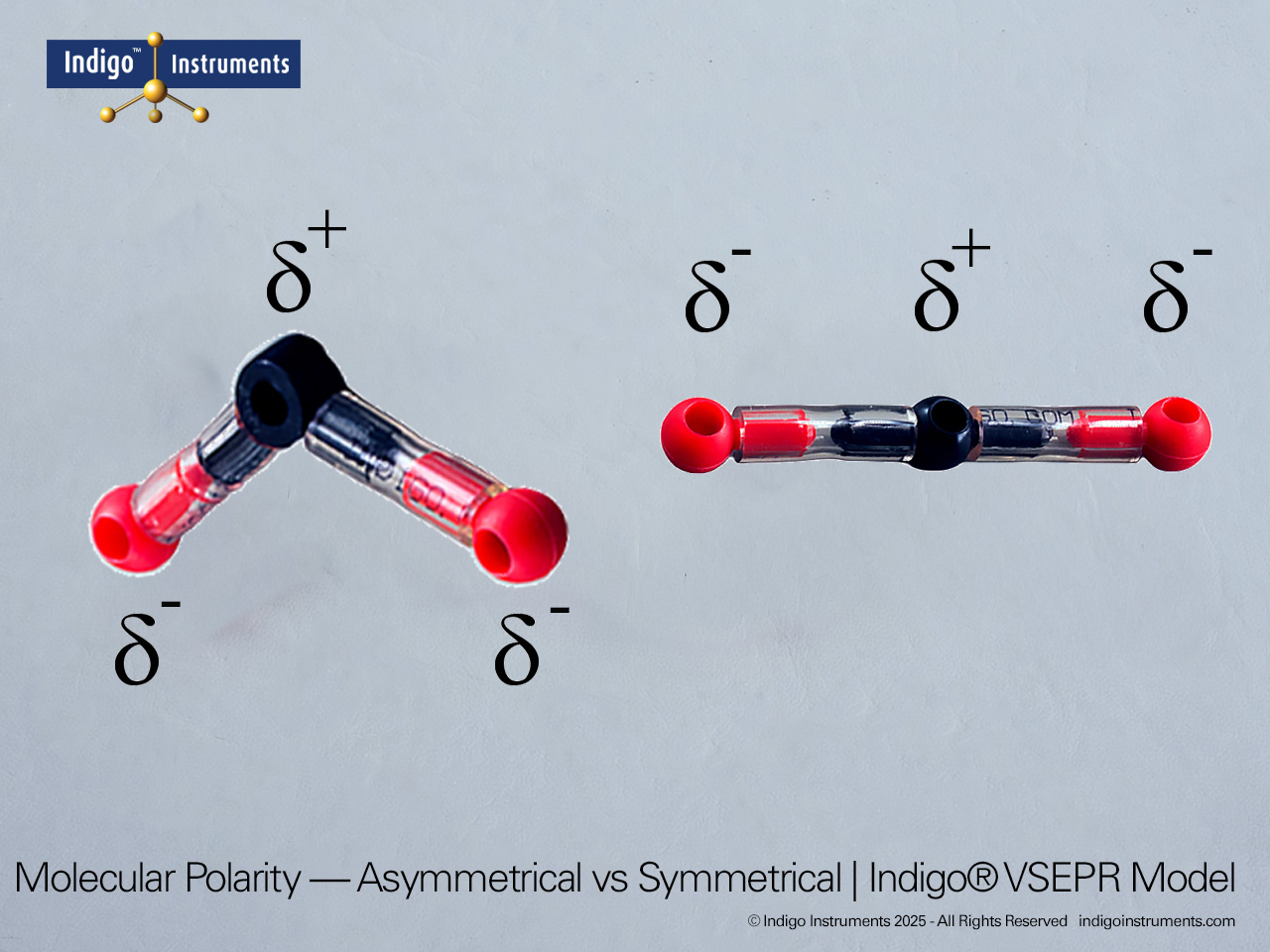
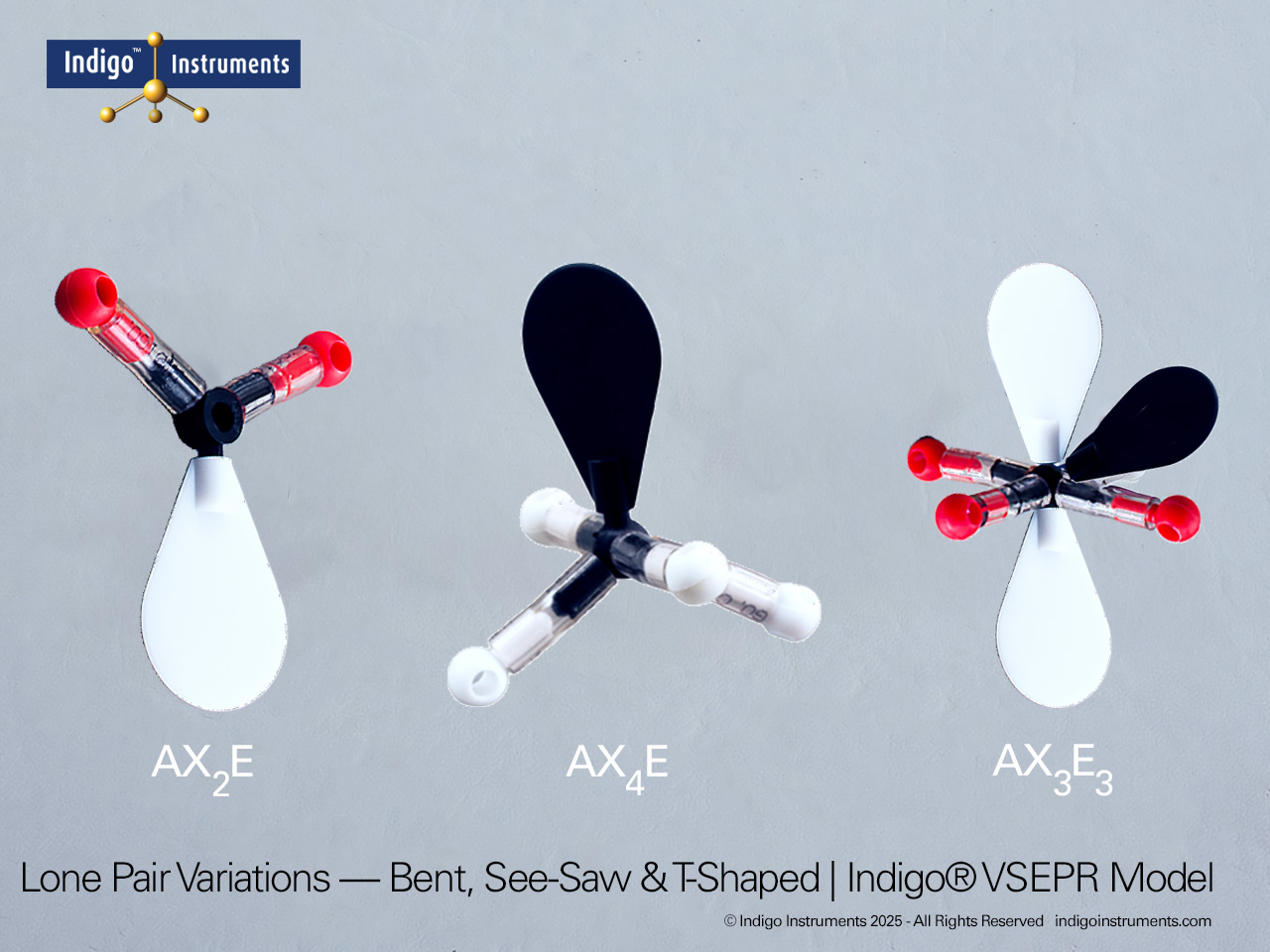
Trigonal Planar Geometry Molecular Model
SKU: 68823W
Explore trigonal planar molecular geometry with the Indigo® VSEPR model kit. Visualize 120° bond angles and electron pair repulsion in AX3 structures.
The Indigo® Molecular Geometry VSEPR Set includes two models that demonstrate trigonal planar geometry. One represents a central atom surrounded by three bonded pairs (AX3), while the other shows two bonded pairs and one lone pair (AX2E). Each model illustrates the 120° bond angles typical of molecules like BF3 and SO3, showing how bonding and lone pairs distribute themselves evenly in a single plane.