Acetylene Molecular Model + Orbitals
SKU: 68823W
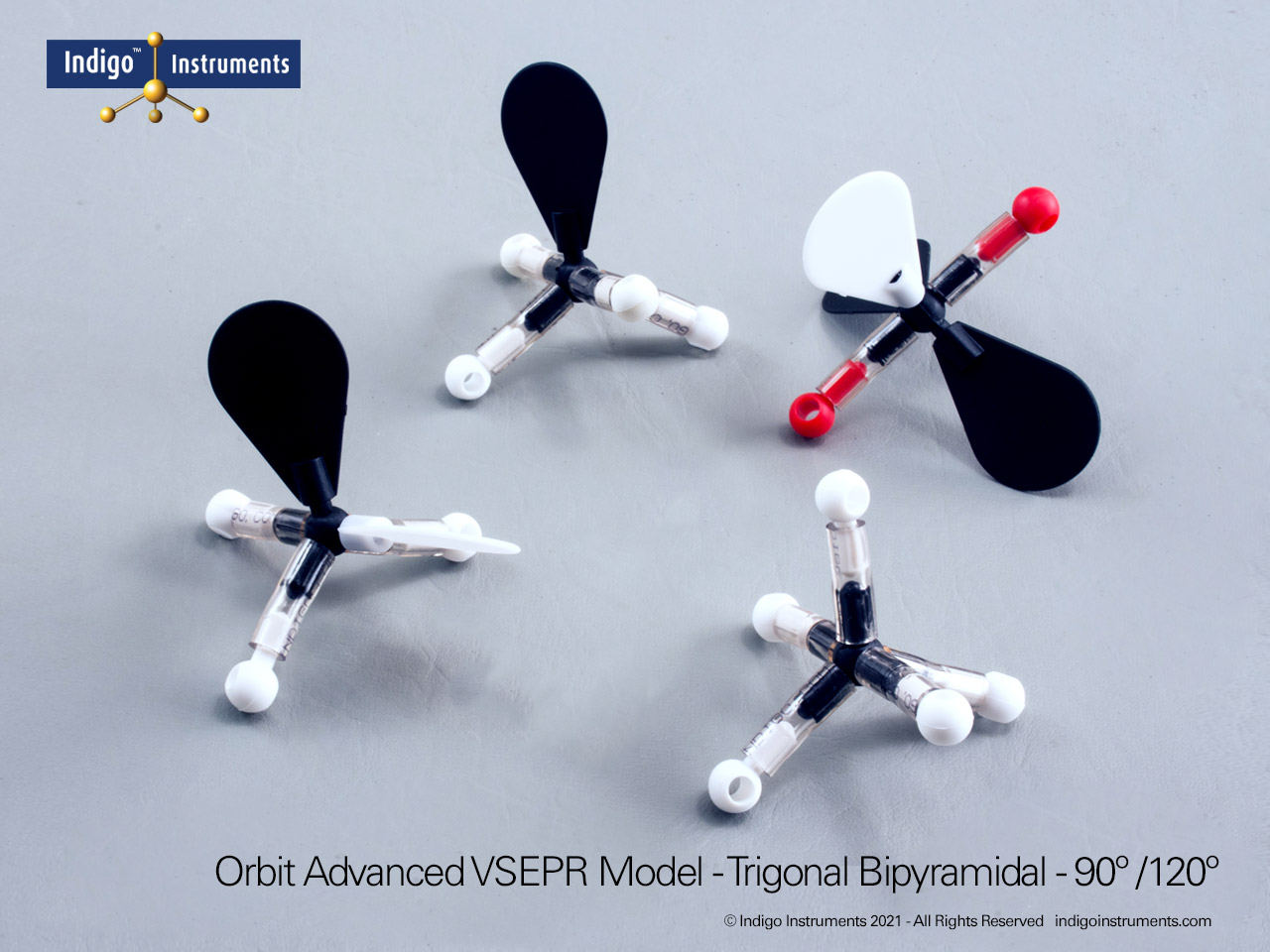
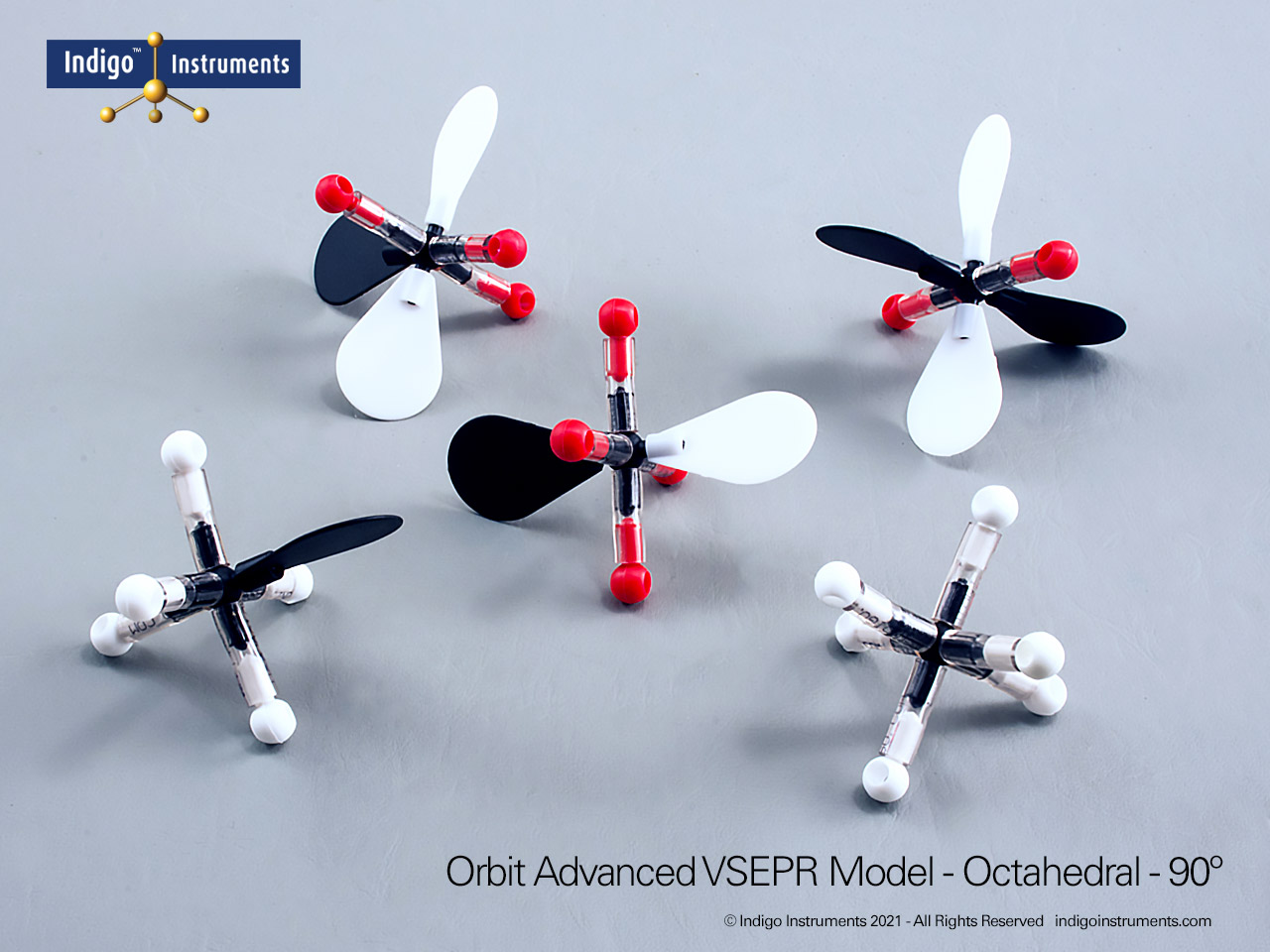
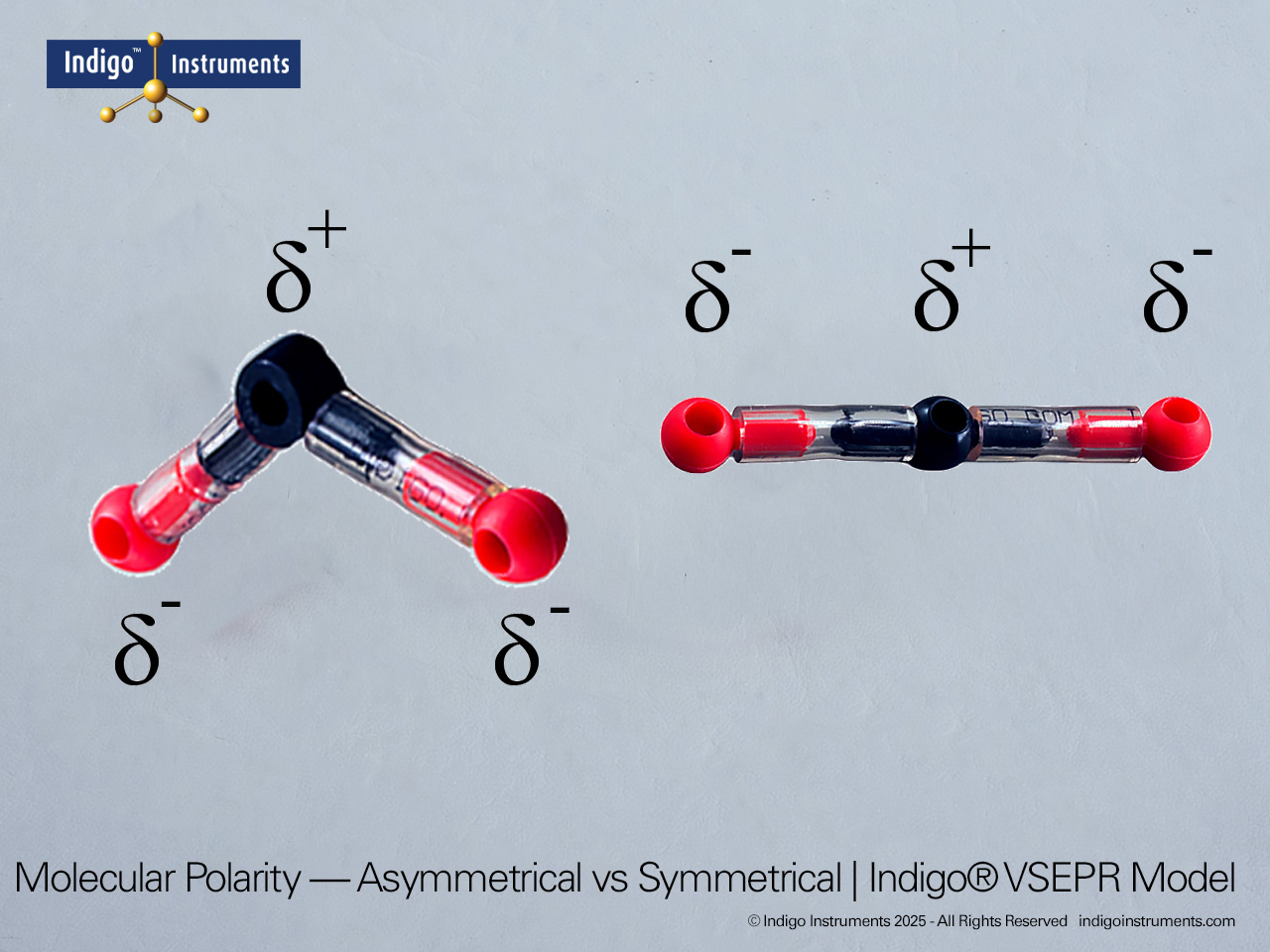
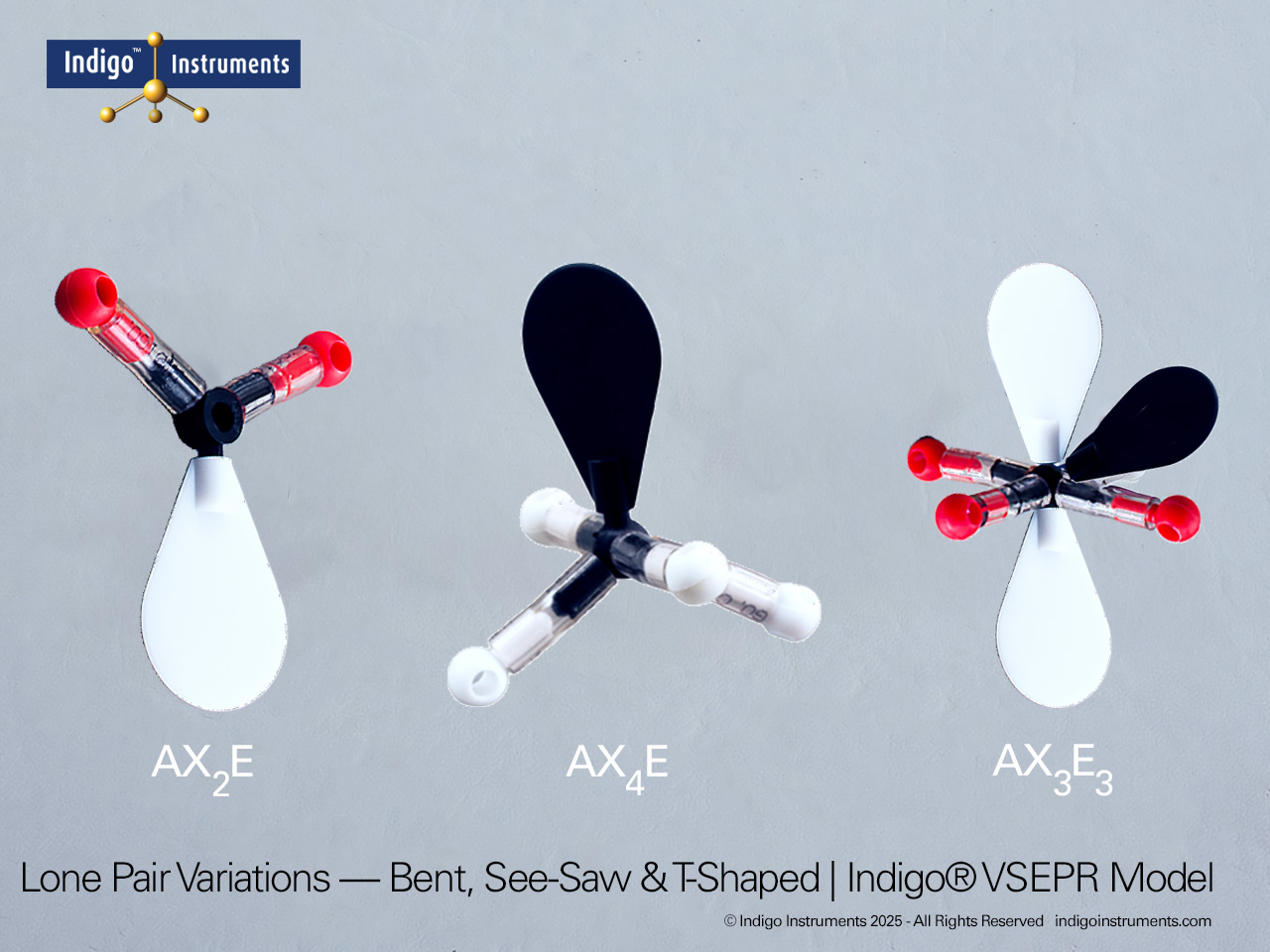
Visualize acetylene (C2H2) with Indigo octahedral atoms and orbitals. Compare molecules of saturated hydrocarbon and unsaturated aliphatic hydrocarbon chains with the Indigo® VSEPR model kit.
Build a molecular model of acetylene (C2H2) using the 4 black octahedral atoms and four white orbitals in the Indigo® VSEPR model kit. This setup allows students to see the linear geometry of the molecule, the triple bond between the carbon atoms, and the sp hybridization of each carbon. Using color-coded orbitals, learners can visualize sigma and pi bond formation and understand electron pair distribution in an unsaturated hydrocarbon.