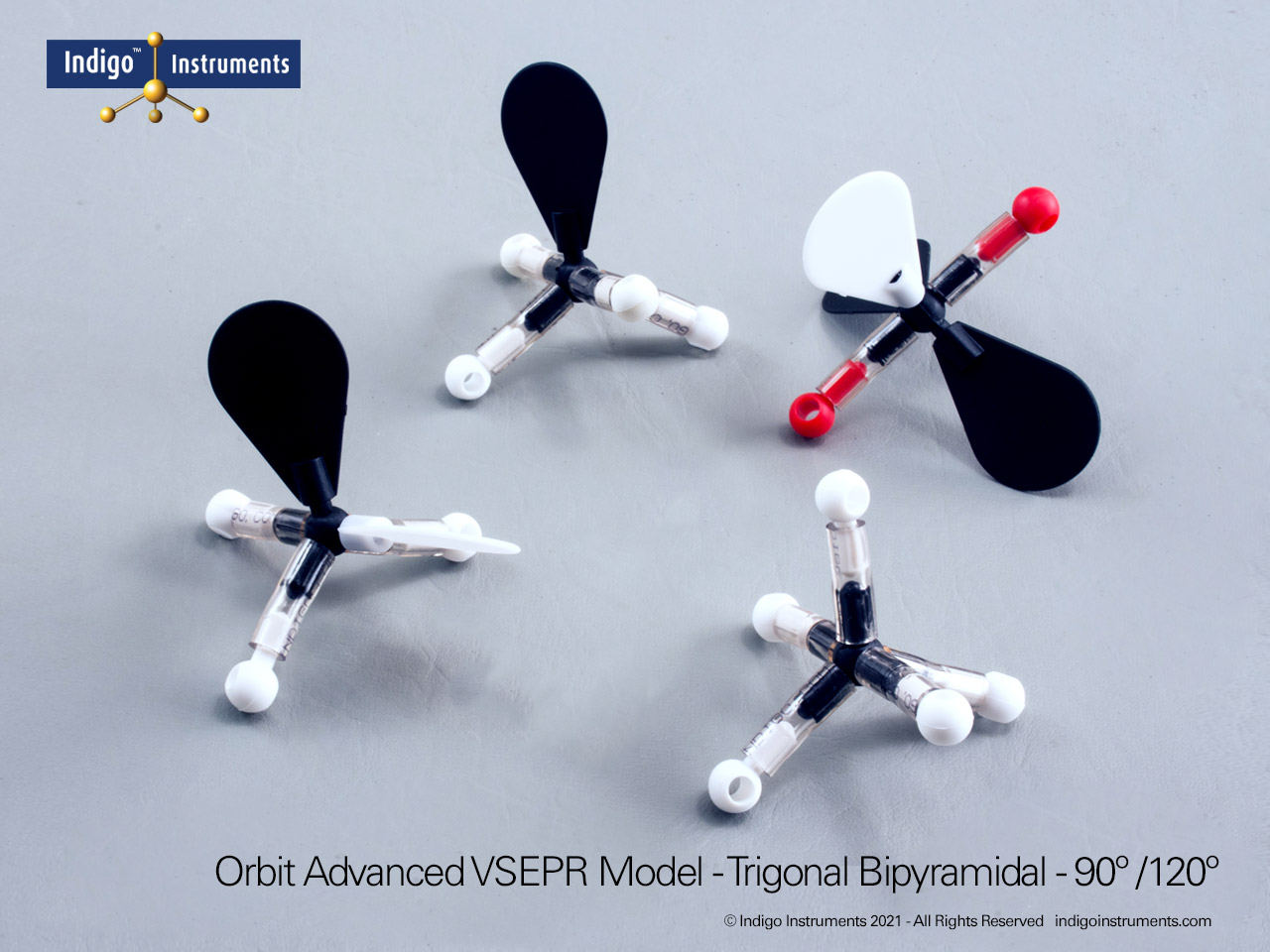
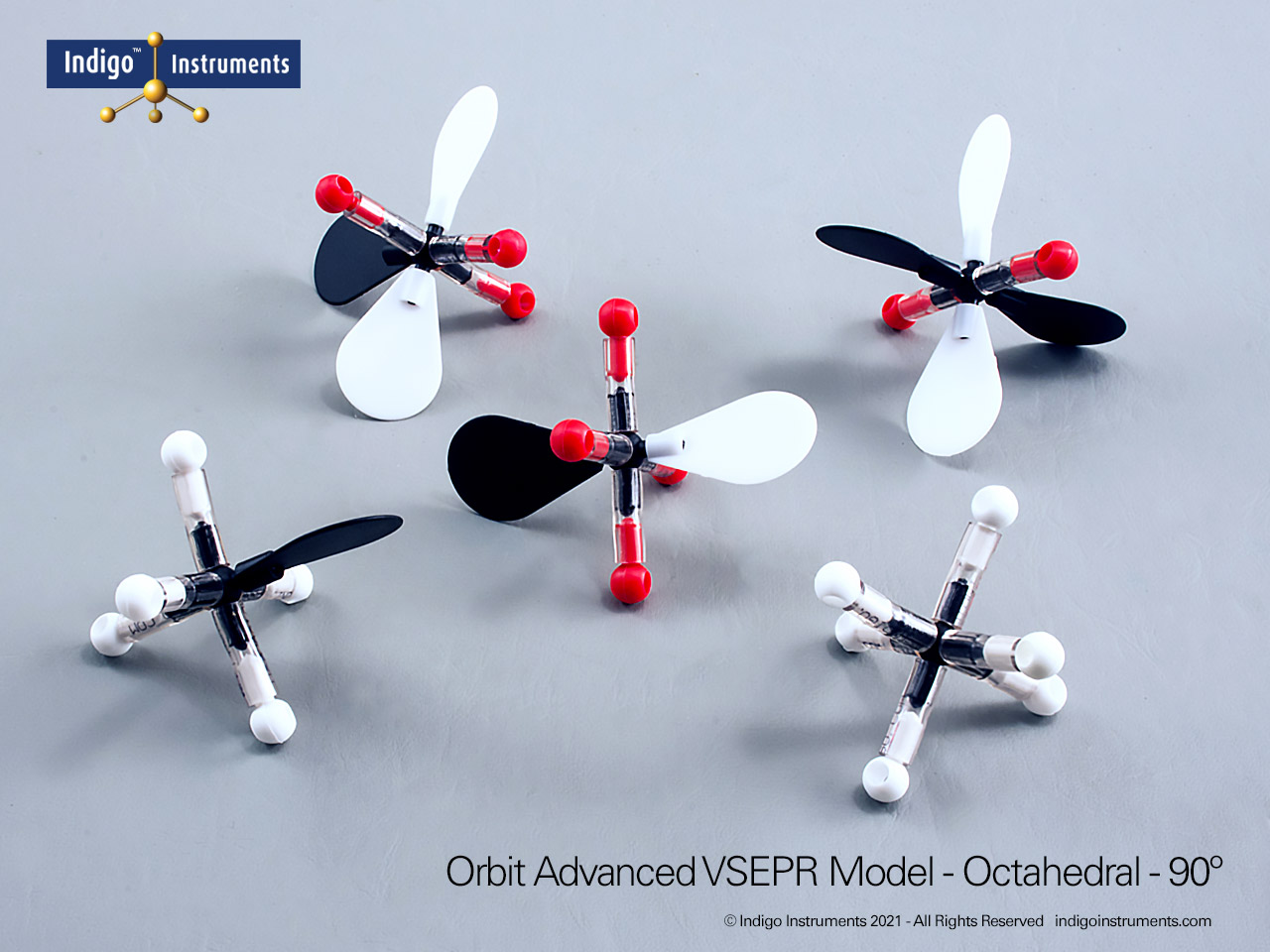
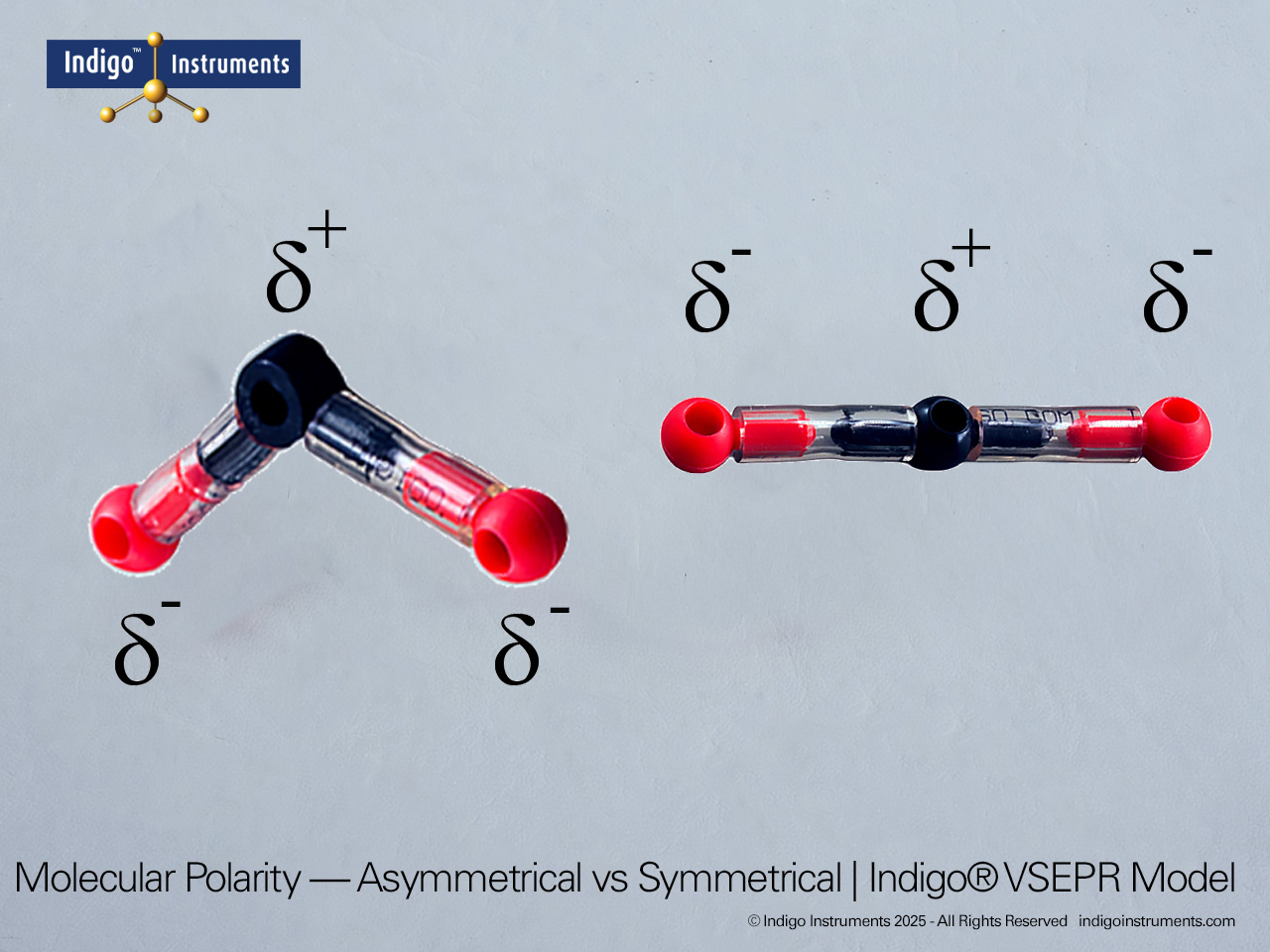
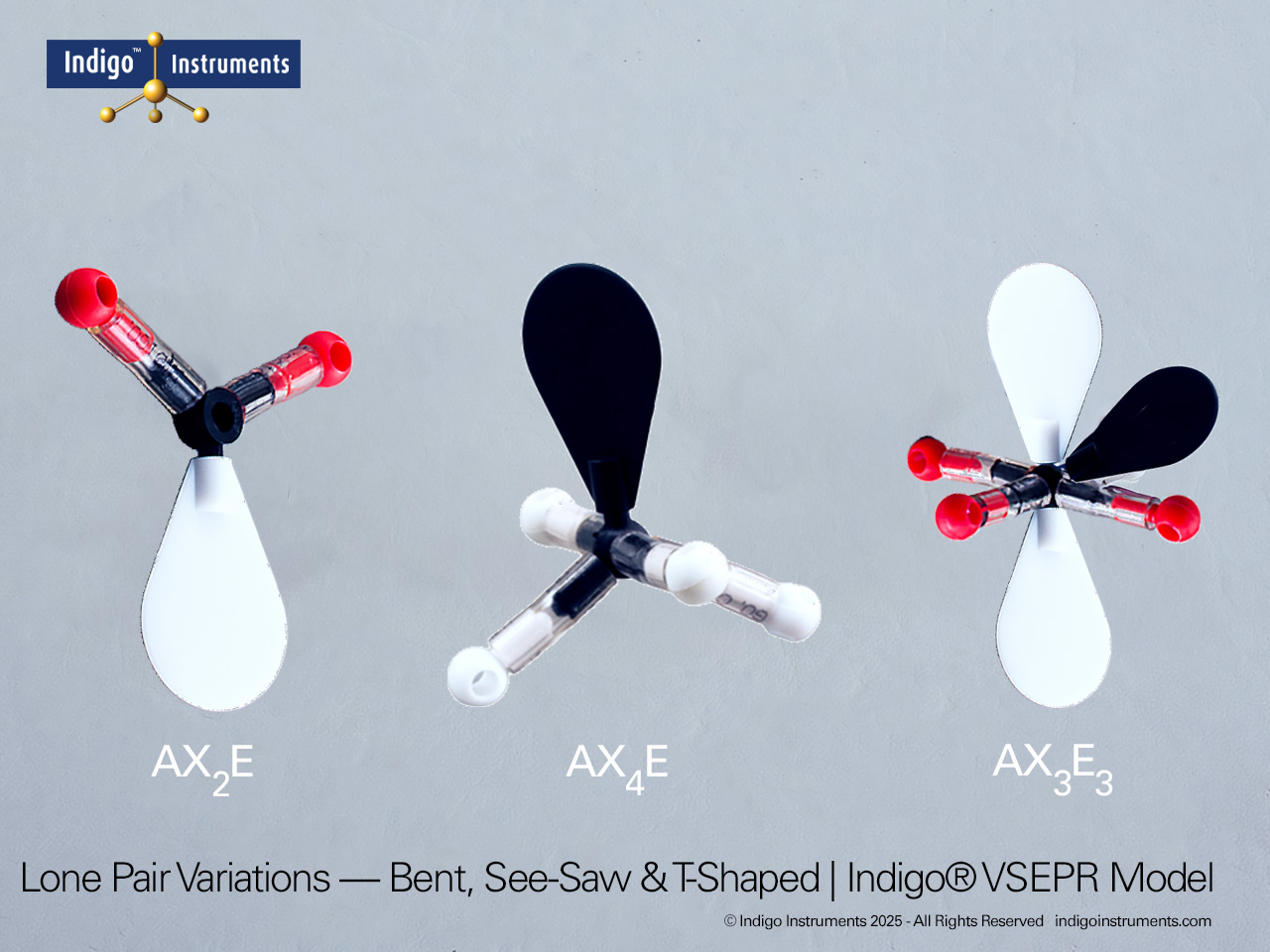
Tetrahedral Geometry Molecular Model
SKU: 68823W
Visualize tetrahedral molecular geometry with the Indigo®’s VSEPR theory model kit. Explore AX4 and lone pair variations to understand bond angles and polarity.
The Indigo® Molecular Geometry VSEPR Set includes three models that demonstrate tetrahedral molecular geometry and its variations. One represents a perfect AX4 configuration with four bonded pairs and 109.5° bond angles, as in CH4 (methane). The second and third illustrate AX3E and AX2E2 arrangements, showing how lone pairs distort angles to form trigonal pyramidal and bent geometries, as seen in NH3 and H2O, respectively.