Octahedral Geometry Molecular Model
SKU: 68823W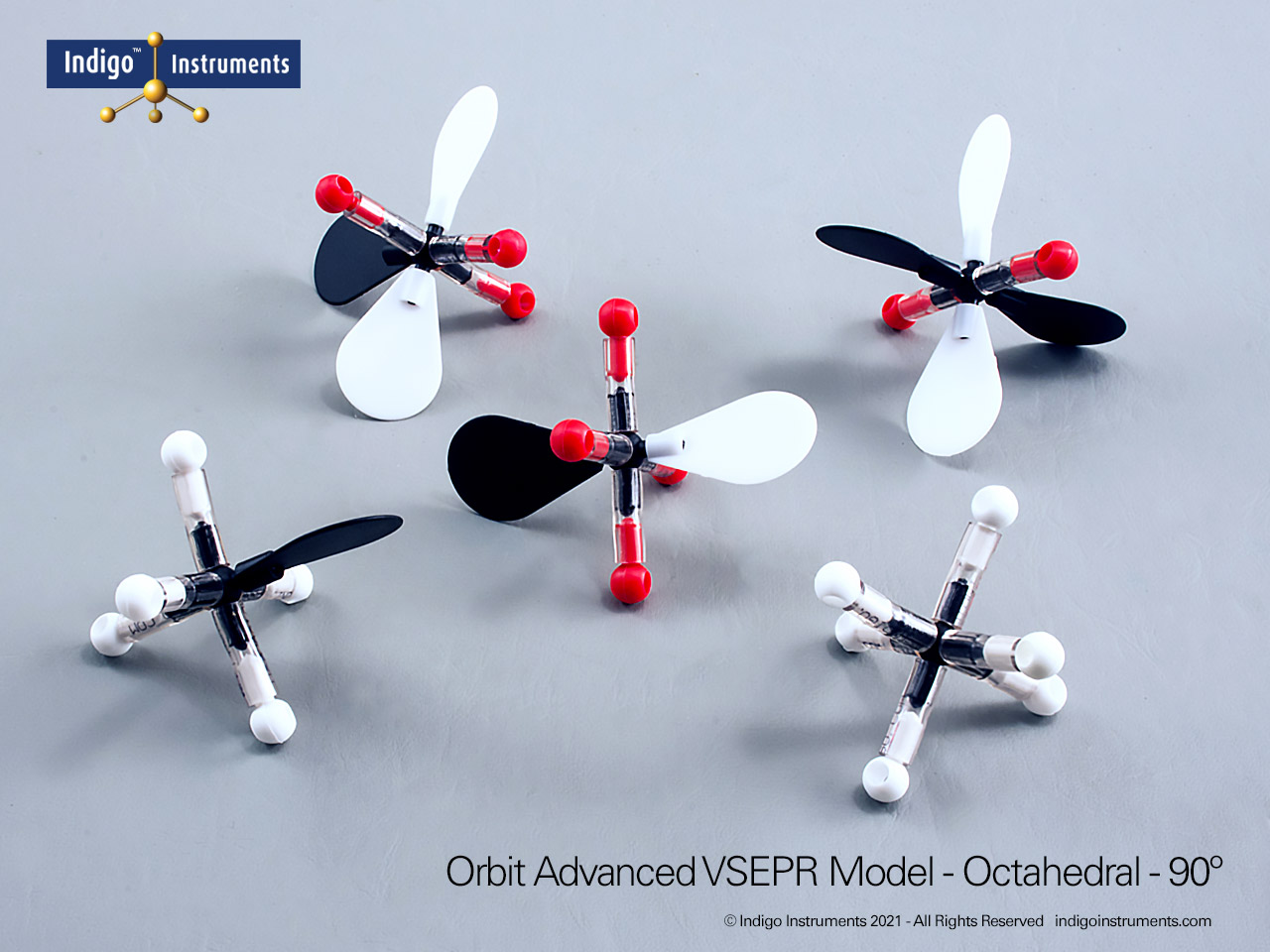
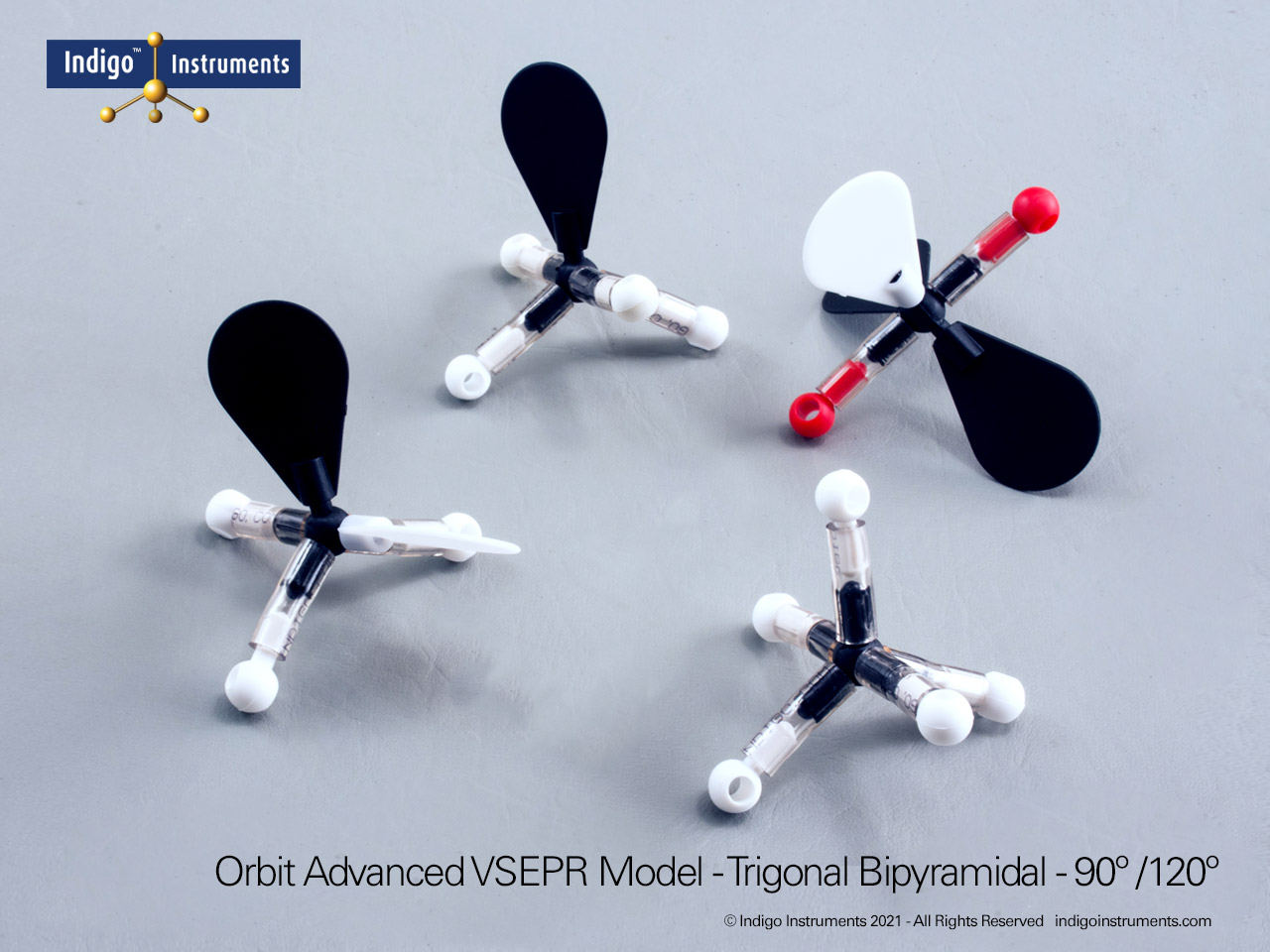
Examine octahedral molecular geometry using the Indigo® VSEPR model kit. Build AX6 structures like SF6 to visualize 90° bond angles and molecular symmetry.
Visualize octahedral molecular geometry with the Indigo® VSEPR theory model kit. Build the AX6 structure to show six bonding pairs arranged symmetrically around a central atom, each separated by 90°. This shape illustrates how six electron domains minimize repulsion to form a perfectly balanced, three-dimensional arrangement.
The Lewis structure of this molecule has octahedral electron geometry which produces VSEPR model shapes with 6 bonded or lone pairs all set 90 degrees apart.