Trigonal Bipyramidal
SKU: 68823W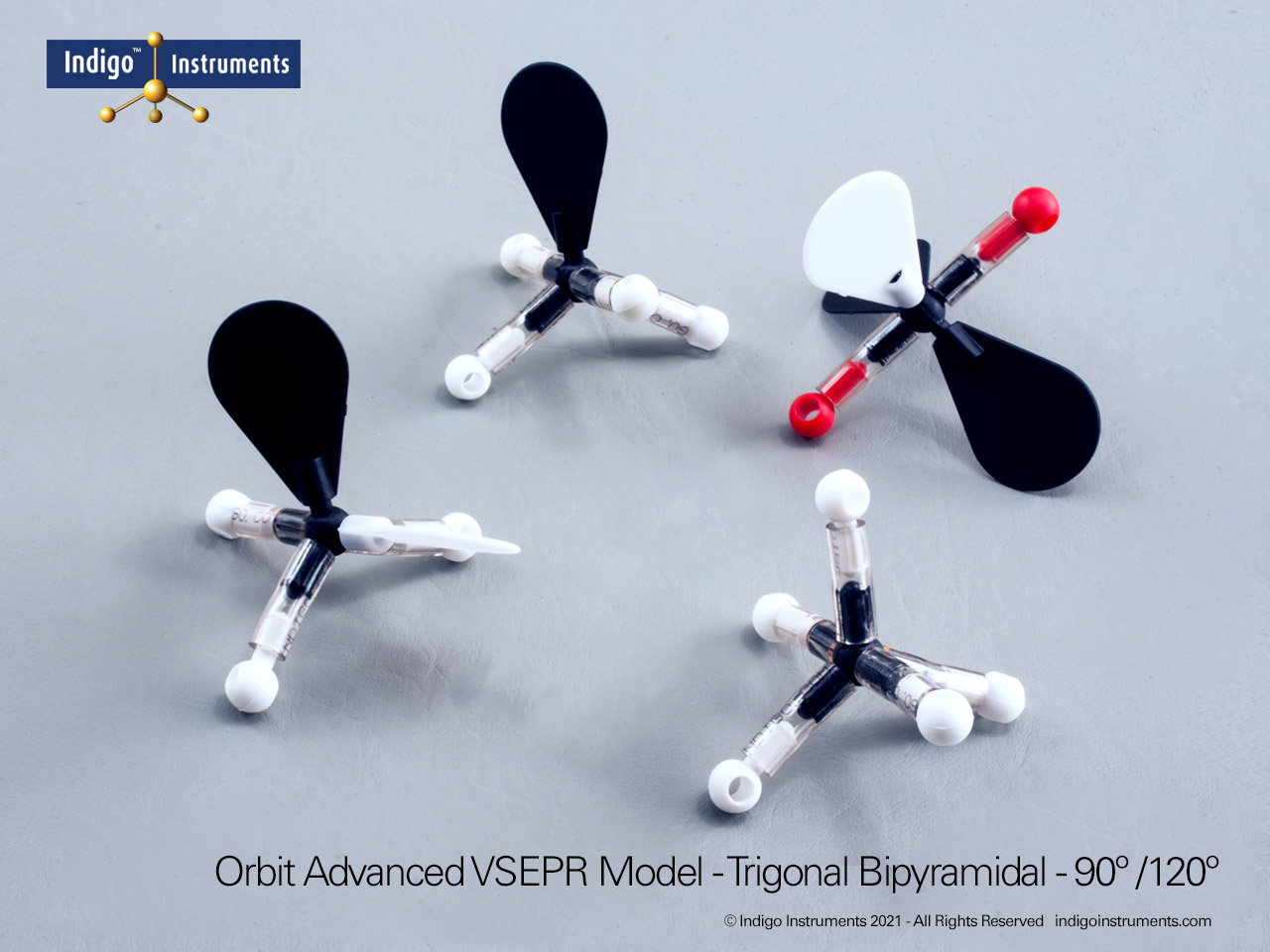
The Indigo® Molecular Geometry Shapes VSEPR Set, only $19.95, includes three models that illustrate trigonal bipyramidal geometry. One has five bonded pairs. The second has 4 bonded pairs and a lone pair. The third has three each bonded and and 2 lone pairs. The red terminal atoms in the indicate they are coplanar with the central atom. All atoms and bonds are autoclavable.
The Lewis structure of this molecule has trigonal bipyramidal electron geometry which produces VSEPR model shapes with 3 120 degree bond angles that are at right angles to a 180 degree pair.