C3 Triphenylphosphine
SKU: 68821W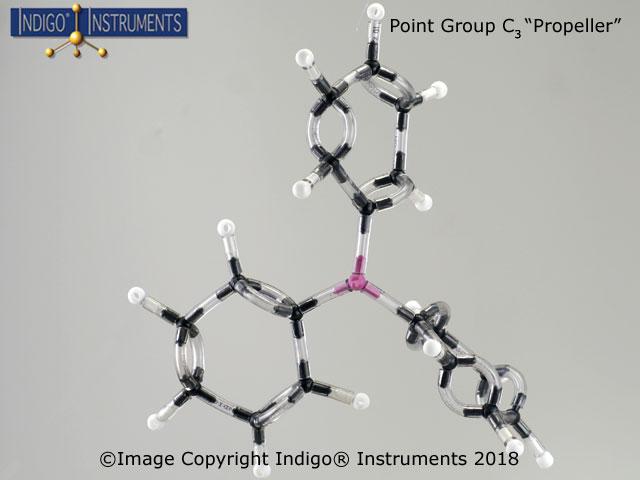
The triphenylphosphine (PPH3) chemical structure shows propeller rotation in the C3 point group. It is a tertiary phosphine, a phosphane where the three hydrogens are replaced by phenyl groups. Triphenylphosphine (PPh3) is trigonal -pyramidal shaped with three phenyl groups around a central phosphorus atom. The 4th position of the tetrahedral N atom has a lone pair of electrons that are not shown (optional in white or black; included in student set 68845NV).
Triphenylphosphine's molecular formula can be shown in two ways: C18H15P or (C6H5)3P.
The twisted nature of the phenyl rings in C3 point group molecule triphenylphosphine only has a C3 axis of rotation. The triphenylphosphine molecule can undergo an E C3 symmetry operation & is an example of a C3 molecular symmetry point group which shows propeller, chiral geometry.
What type of ligand is triphenylphosphine? Triphenylphosphine is typically classified as a monodentate ligand and can be used as a reducing agent in organic synthesis & in transition metal complexes such as catalysts in organometallic chemistry.










![D3 [Co(en)3]3+ D3 [Co(en)3]3+](/images/products-resp/point-group-d3-68821w.jpg)











Thanks for the feedback. It is an unusual set & the only one we know of that can build ferrocene.