Phenolphthalein
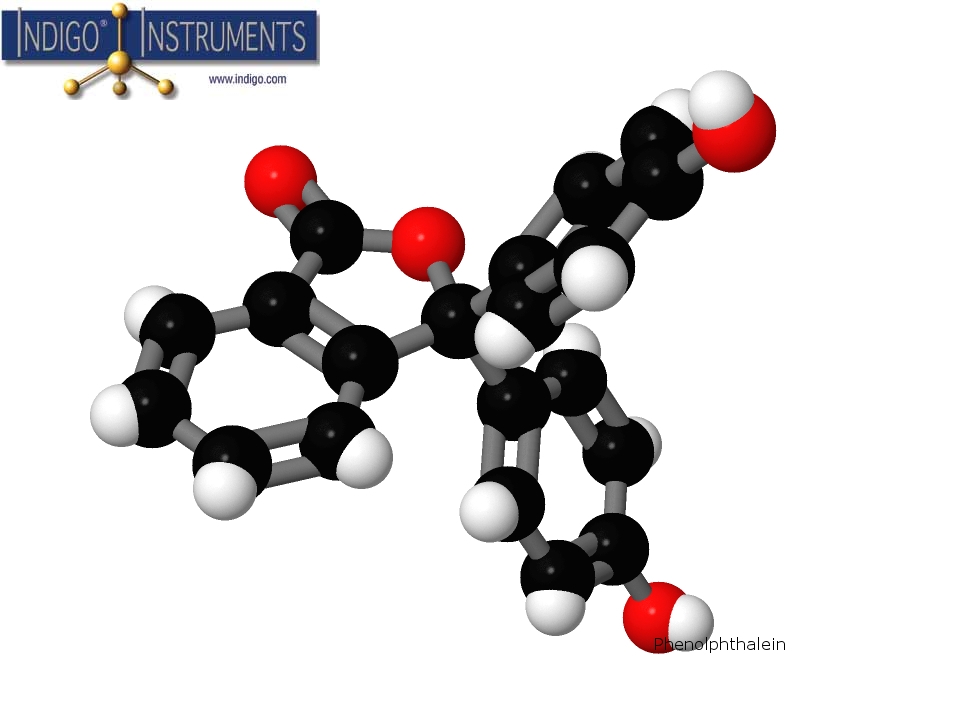
Build a molecular structure model of the pH indicator Phenolphthalein using Genuine Molymod Model Parts by Indigo
What is Phenolphthalein?
As an indicator, phenolphthalein's pH range is between 8.2-12 where it changes from colorless to pink/fucshia. In very acidic solutions near pH 0 phenolphthalein appears orange.
Phenolphthalein's uses include the Kastle-Meyer test for blood where the presence of hemoglobin causes the indicator solution to turn pink.
The phenolphthalein structure is a is good example of a C3 Point Group & shows "propeller" rotation. It was discovered in 1871 by German chemist Adolf von Baeyer & was commonly used as laxative until it was discovered to be a carcinogen.
A molecular model of Phenolphthalein in the Molymod Hybrid Dome style uses the atoms & bonds in the parts list below. Our free 3D Molecular Model Builder shows other styles & allows for image rotation as an assembly guide.
| Molecular Weight | Chemical Formula |
|---|---|
| 318.3282 | C20H14O4 |