Permethrin
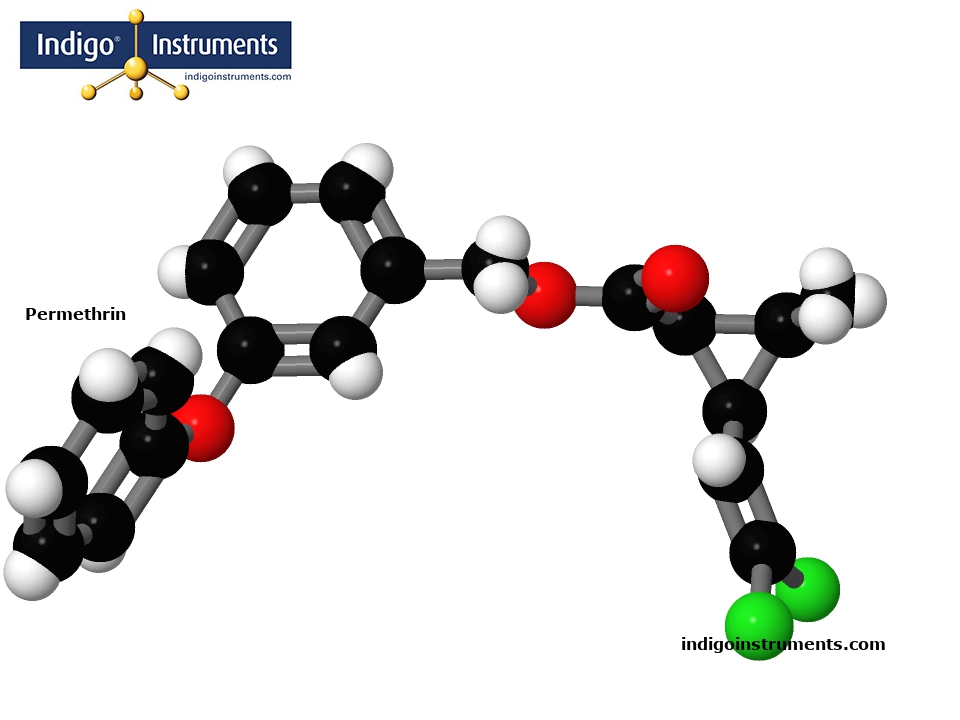
Assemble a molecular structure model of Permethrin from Authentic Molymod Model Parts by Indigo.
Permethrin is classed as a synthetic neurotoxic pyrethroid insecticide that targets a wide variety of insects. It is a synthetic chemical compound based on extracts from the chrysanthemum flower. Permethrin control mechanism of arthropods such as insects & ticks is depolarization of sodium transport channels in neuronal membranes to cause paralysis.
Permethrin's chemical structure is a cyclopropanecarboxylate ester based on a cyclopropane ring. It has four stereoisomers with the (1R,3S)-trans and (1R,3R)-cis enantiomers responsible for the insecticidal properties of permethrin.
Common Uses for Permethrin:
- Food and Feed Crops
- Mosquito Control
- Lawns and Gardens
- Livestock and Pets
- Medications - Scabies and head lice
- Spraying buildings, clothing
- Flea Collars
- Restaurants
- Tick Repellant
A molecular model of Permethrin in the Molymod Hybrid Dome style uses the atoms & bonds in the parts list below. Our free 3D Molecular Model Builder shows other styles & allows for image rotation as an assembly guide.
| Molecular Weight | Chemical Formula |
|---|---|
| 391.2932 | C21H20Cl2O3 |