Kynurenine
Construct a 3D molecular structure model of Kynurenine using Authentic Molymod Atoms & Bonds by Indigo
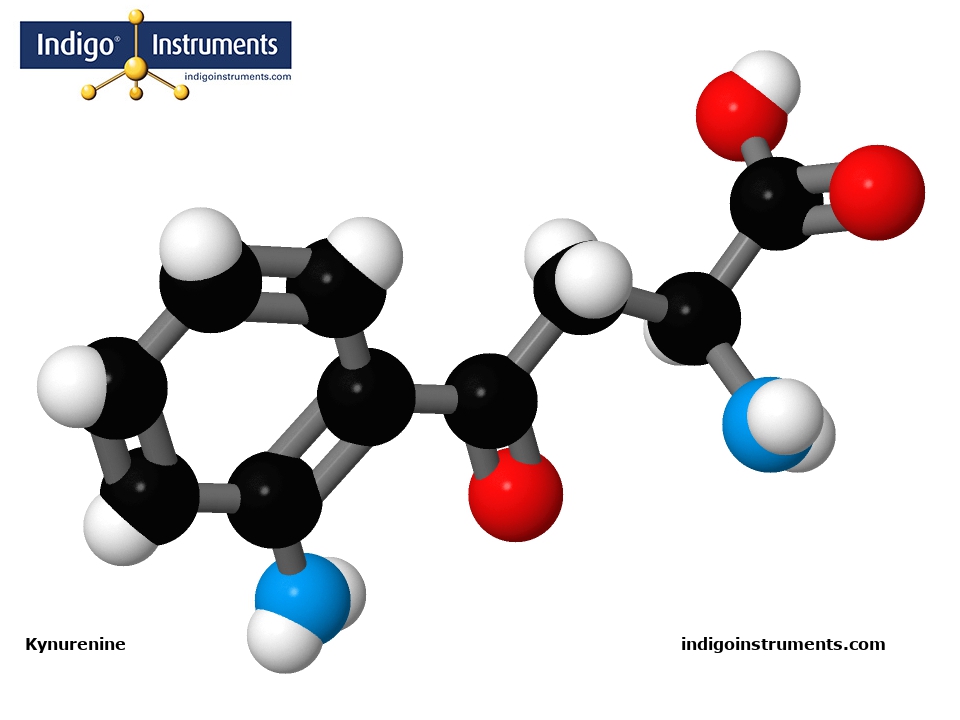
Kynurenine is an aromatic α-amino acid derivative as defined by its carboxyl (-COOH) and amino (-NH2) groups and indole-like ring system. It does have the bicyclic structure of its metabolic precursor, the essential amino acid tryptophan.
Kynurenine is a metabolic intermediate in the tryptophan degradation pathway which leads to the production of nicotinamide adenine dinucleotide (NAD), an essential coenzyme in cellular energy metabolism. Tryptophan is converted to N-formylkynurenine by the enzyme indoleamine 2,3-dioxygenase (IDO). N-formylkynurenine then loses its formyl group to yield free kynurenine. Kynurenine branches into several pathways but arguably the most important are the final steps that lead to NAD. synthesis: 3-Hydroxykynurenine → 3-Hydroxyanthranilic Acid → Quinolinic Acid → NAD, which is critical for cellular respiration, energy metabolism, and DNA repair.
Some sources claim that tryptophan in banana peels can be extracted simply by boiling them & that the "tea" made from the brew can be of help in alleviating issues related to sleep deprivation.
A molecular model of Kynurenine in the Molymod Hybrid Dome style uses the atoms & bonds in the parts list below. Our free 3D Molecular Model Builder shows other styles & allows for image rotation as an assembly guide.
| Molecular Weight | Chemical Formula |
|---|---|
| 208.2164 | C10H12N2O3 |