EDTA
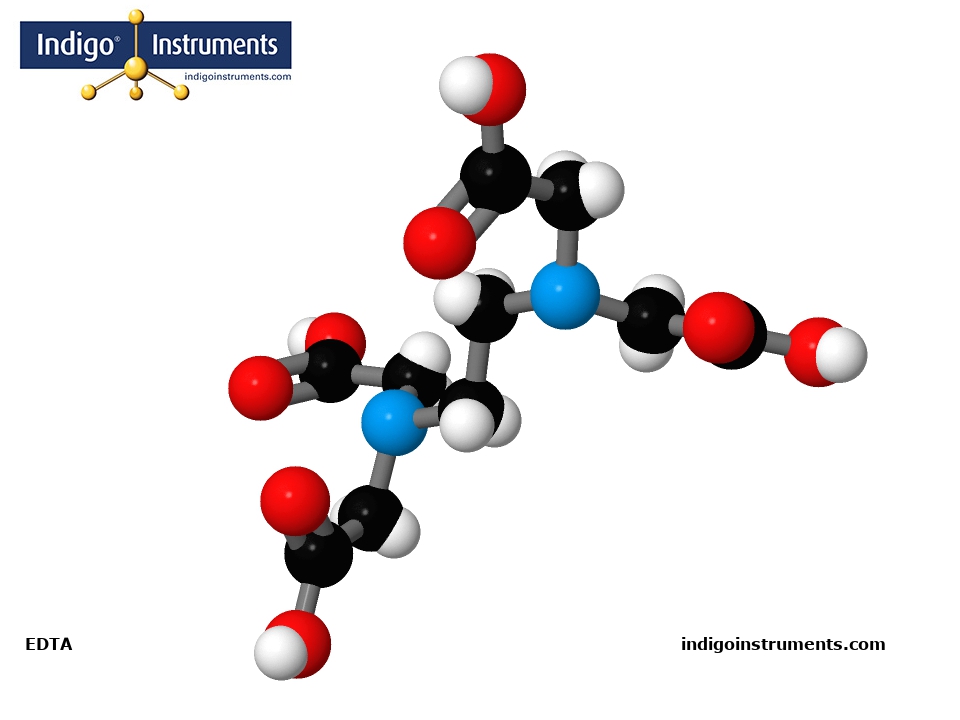
Fabricate a 3D molecule model of the chelating agent EDTA (Ethylenediaminetetraacetic acid) using Authentic Molymod Model Parts by Indigo.
EDTA (Ethylenediaminetetraacetic acid) has a chemical structure described as an amino-polycarboxylic acid. It is an important ligand & chelating material commonly used to remove limescale caused by minerals such as calcium carbonate and forms complexes that precipitate out of water onto metal pipes, glass & other surfaces. It also forms water-soluble complexes even at neutral pH to deliver iron ions under conditions where iron oxides are insoluble.
Most students will utilize EDTA in their introductory inorganic labs and learn about it through point group theory as it is the ligand commonly used to show D3 point group. EDTA is a common bidentate ligand.
A molecular model of EDTA in the Molymod Hybrid Dome style uses the atoms & bonds in the parts list below. Our free 3D Molecular Model Builder shows other styles & allows for image rotation as an assembly guide.
| Molecular Weight | Chemical Formula |
|---|---|
| 292.245 | C10H16N2O8 |