Salt in Food Preservation
SKU: 68791W
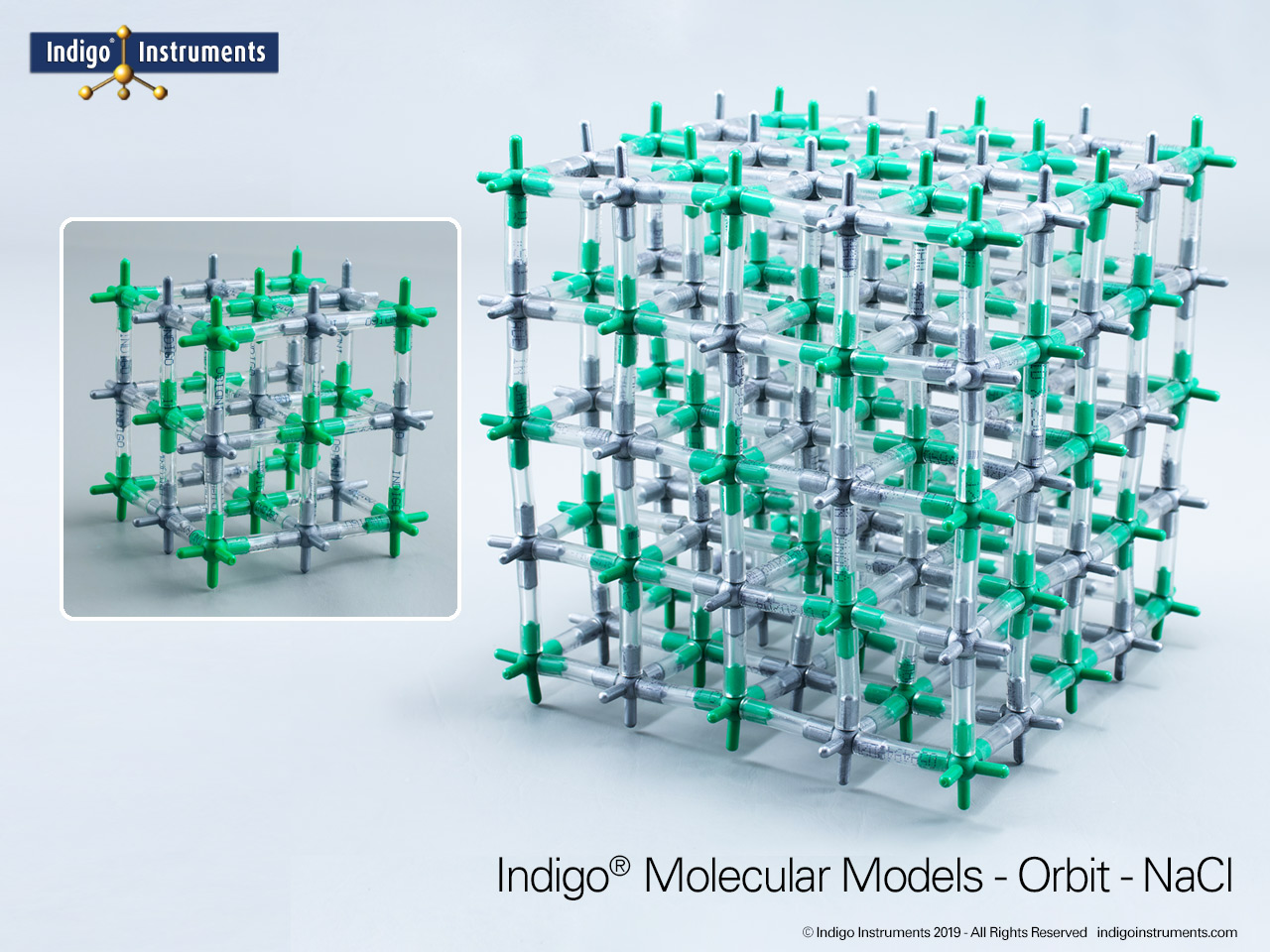
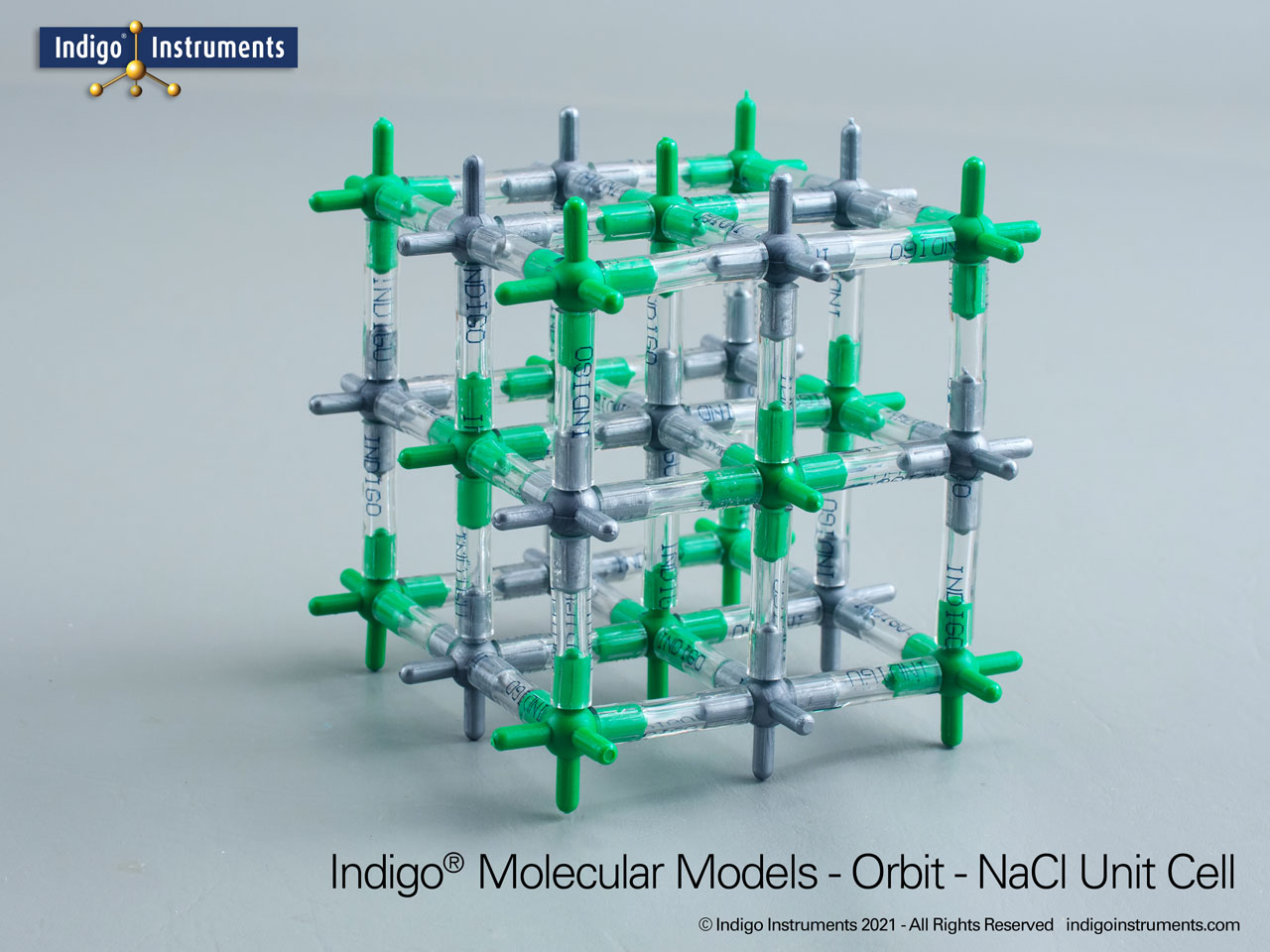
Use the Indigo® NaCl crystal lattice model to ponder how sodium chloride preserves food by osmotic pressure and inhibits microbial growth.
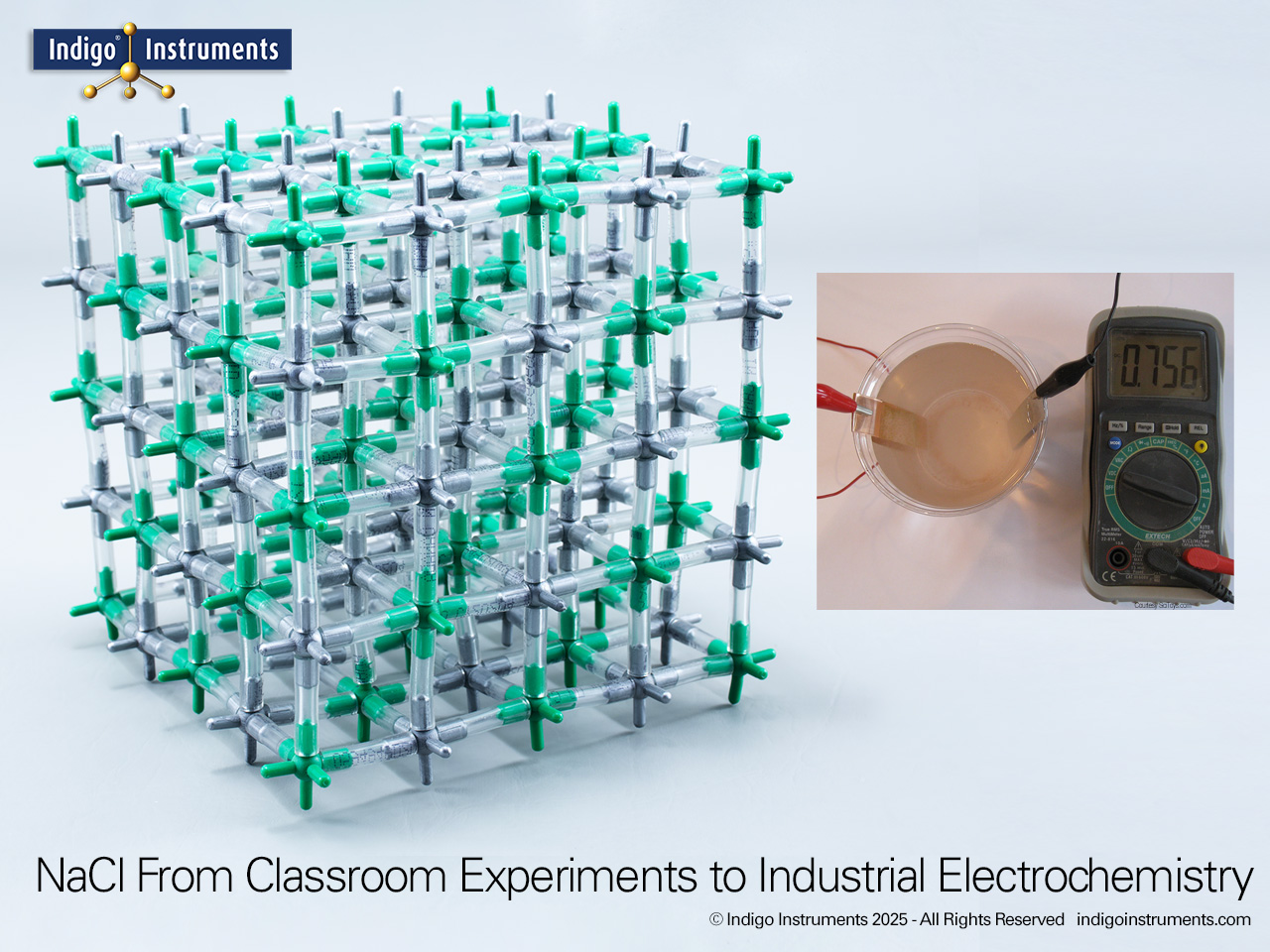
Sodium chloride (NaCl) is the oldest and most universal food preservative. Long before refrigeration, salting was essential to keep fish, meat, and vegetables safe for transport and storage. The chemistry of salt food preservation lies in its ability to lower water activity, creating osmotic pressure that draws water out of microbial cells. This NaCl antimicrobial mechanism prevents bacteria, yeasts, and molds from thriving, extending shelf life. Today, food science courses still use NaCl as the classic case study linking ionic solubility, osmosis, and microbial inhibition.
Indigo Instruments has maintained a substantial inventory of genuine Cochranes of Oxford (Orbit) parts for 30+ years (scroll down to see "Skeletal (Orbit/Minit) and are compatible with every molecular model kit we have sold since day 1. This level of quality may appear expensive but no parts support from other vendors costs even more.