Zeolite Y Gas Separation
SKU: 68784W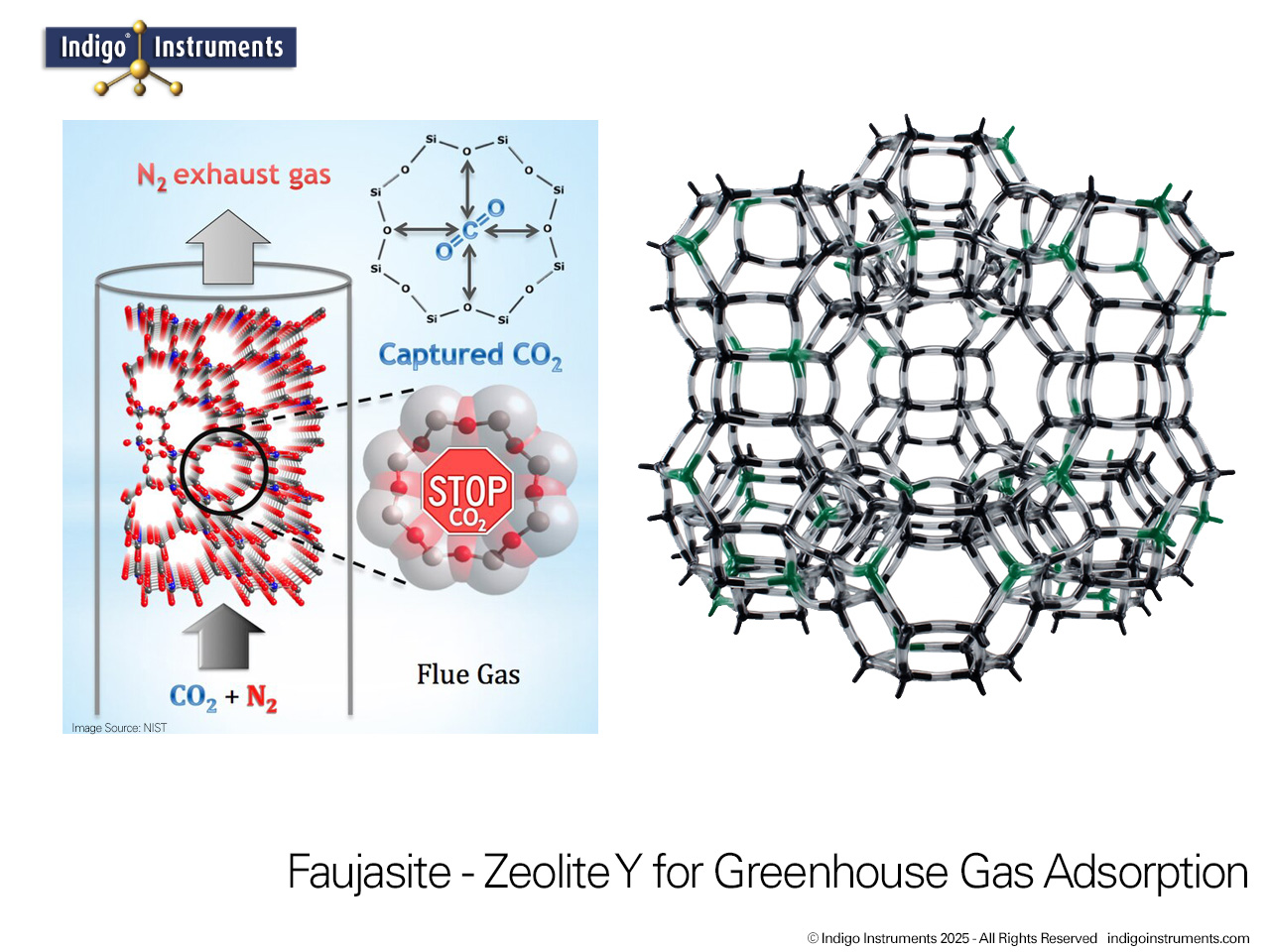
Use this Indigo® Zeolite Y molecular for teaching about gas separation and molecular sieving. Learn how pore size and shape enable selective adsorption of CO2, N2, O2, and other gases, with classroom and research examples.
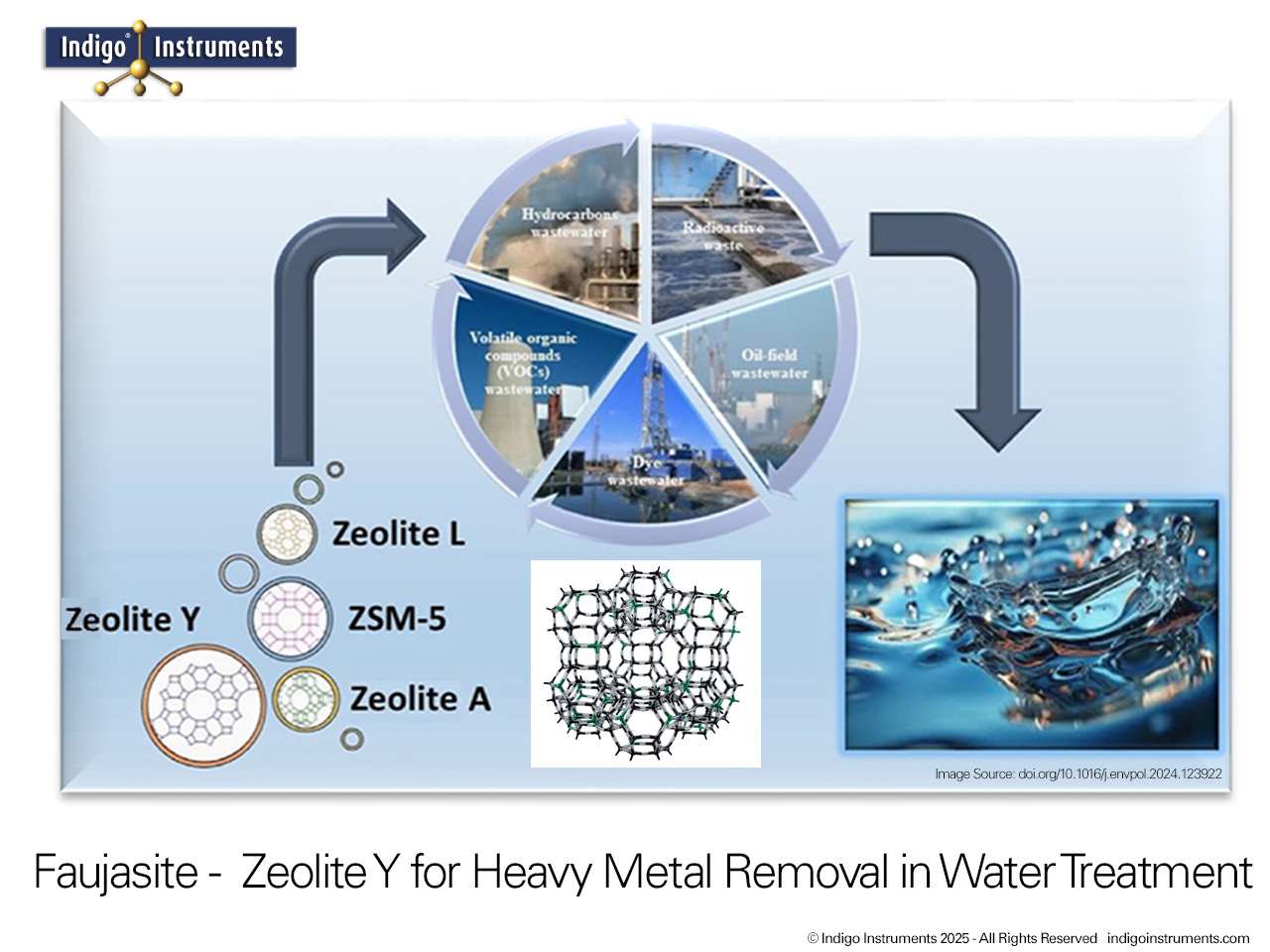
Zeolite Y, a synthetic faujasite, is widely used as a molecular sieve for selective gas adsorption and separation. Its uniform 12-membered ring pores and high surface area allow it to discriminate between molecules based on size, shape, and polarity. This makes Zeolite Y an ideal teaching tool for chemistry and chemical engineering students to visualize gas separation principles in a tangible way. By modeling the interactions of gases such as O2, N2, CO2, and H2 within the zeolite framework, students can observe how kinetic diameter, polarity, and diffusion constraints influence adsorption. Beyond the classroom, Zeolite Y serves as a model for environmental and industrial applications, including carbon capture, air purification, and selective gas separation processes, providing a clear connection between molecular structure and real-world functionality.
Indigo Instruments has maintained a substantial inventory of genuine Cochranes of Oxford (Orbit) parts for 30+ years (scroll down to see "Skeletal (Orbit/Minit) and are compatible with every molecular model kit we have sold since day 1. This level of quality may appear expensive but no parts support from other vendors costs even more.

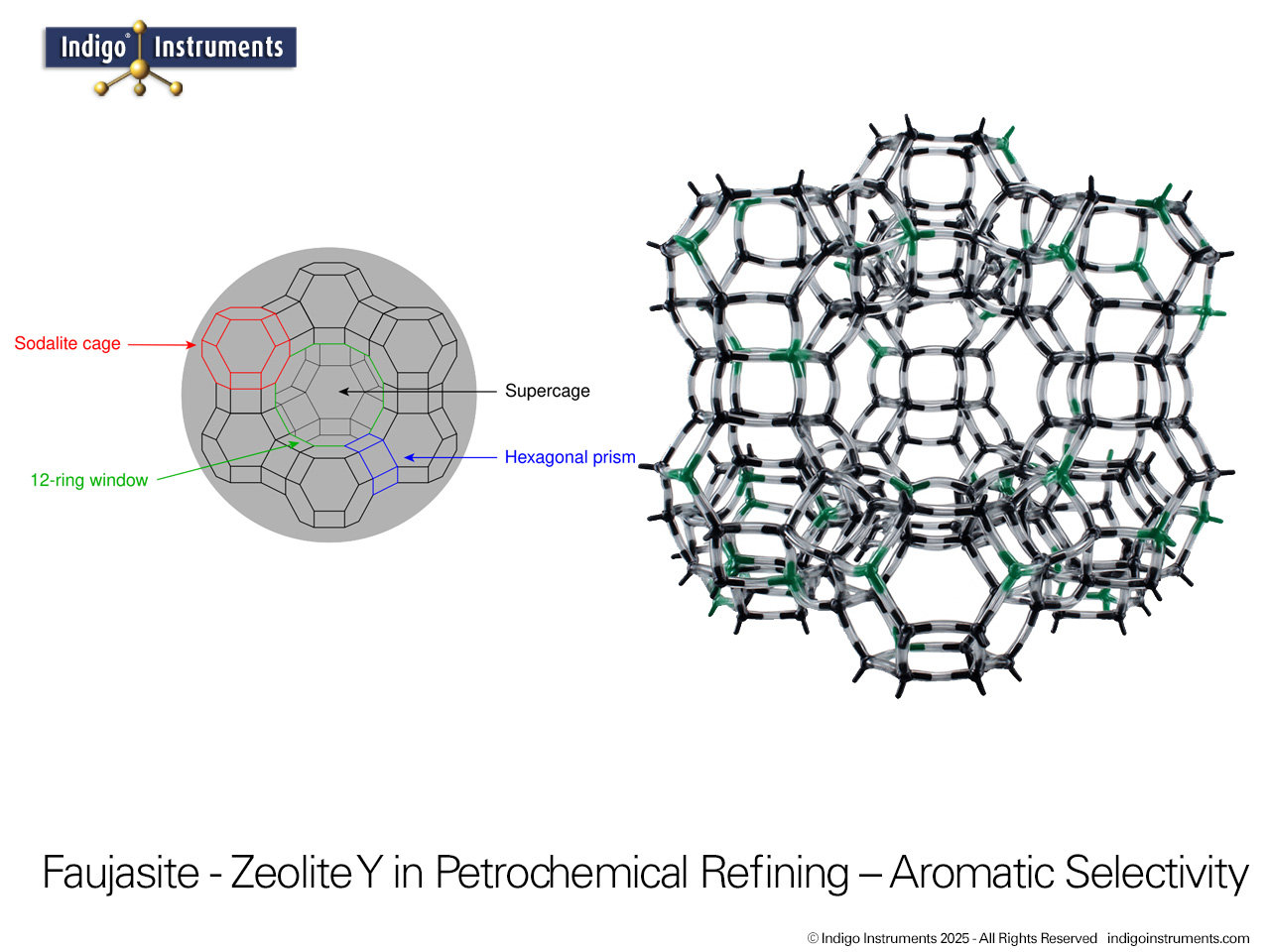
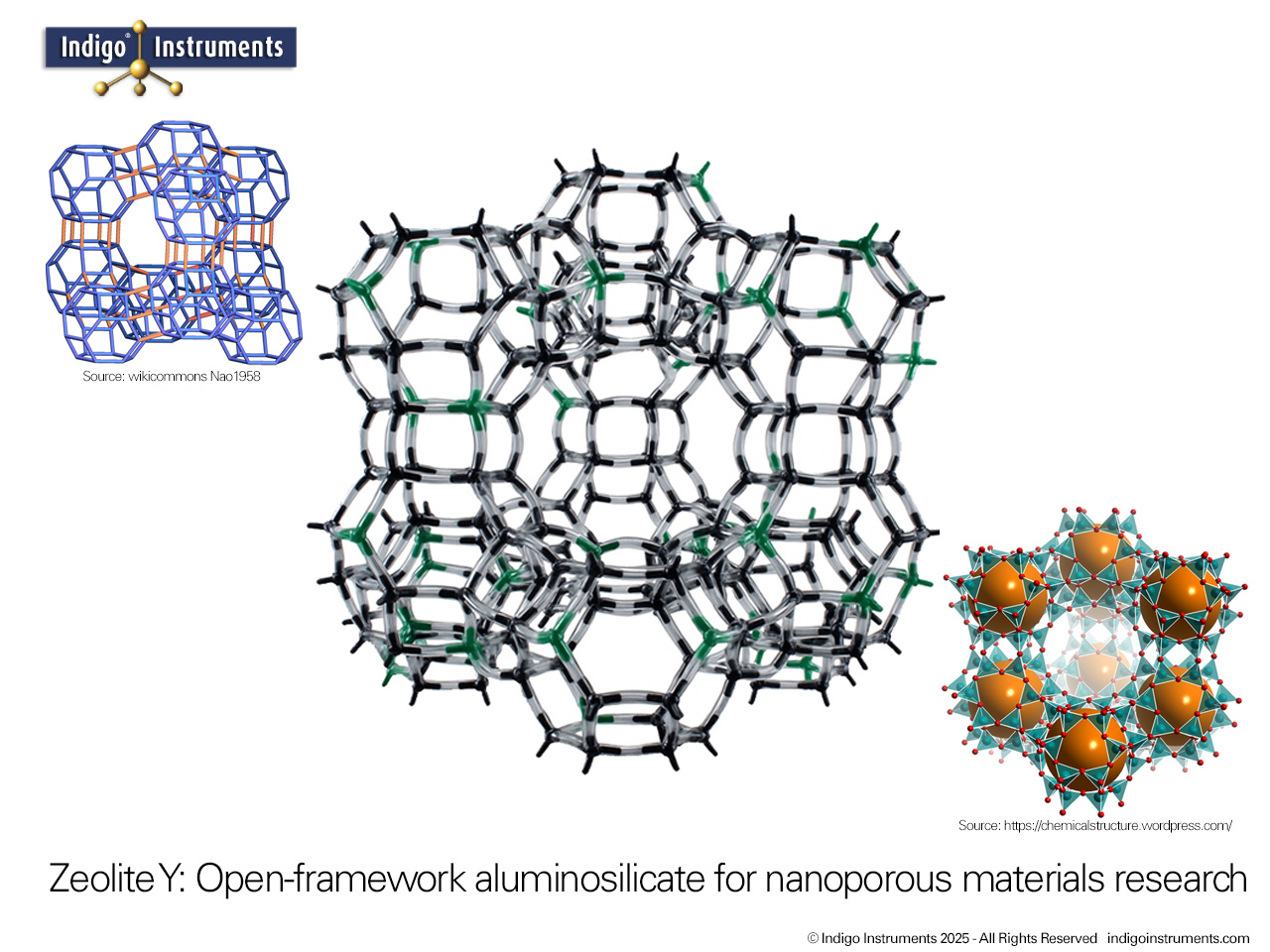



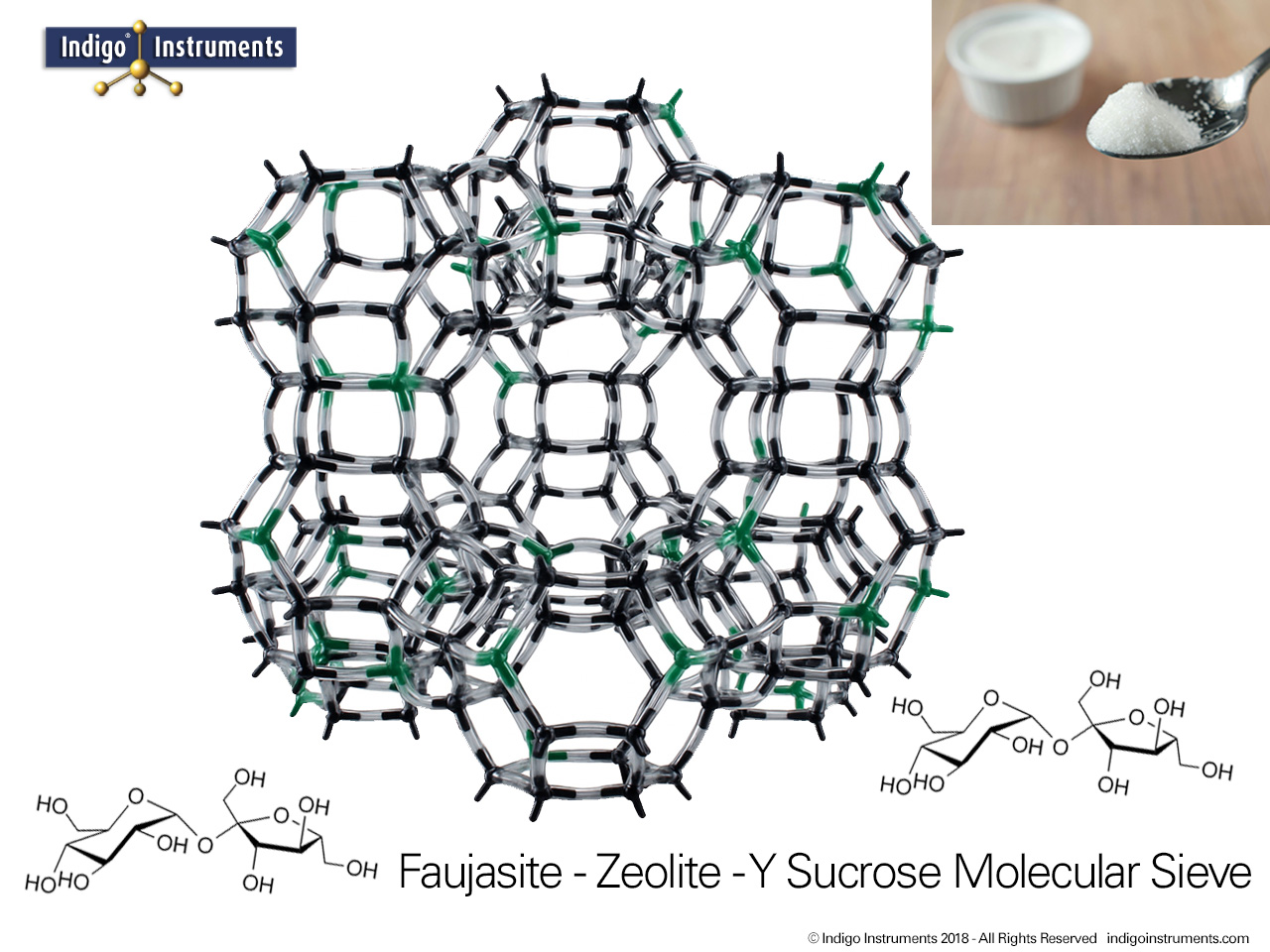
Thanks for the feedback. Trying to describe the assembly was next to impossible so we'll get a picture done of the sodalite cage & add that shortly. Here's a bit more on this model also used for teaching: Zeolite Catalyst Molecular Models Teach Oil Cracking