Reducing Sugars and Amino Acids
SKU: 33814-GluP
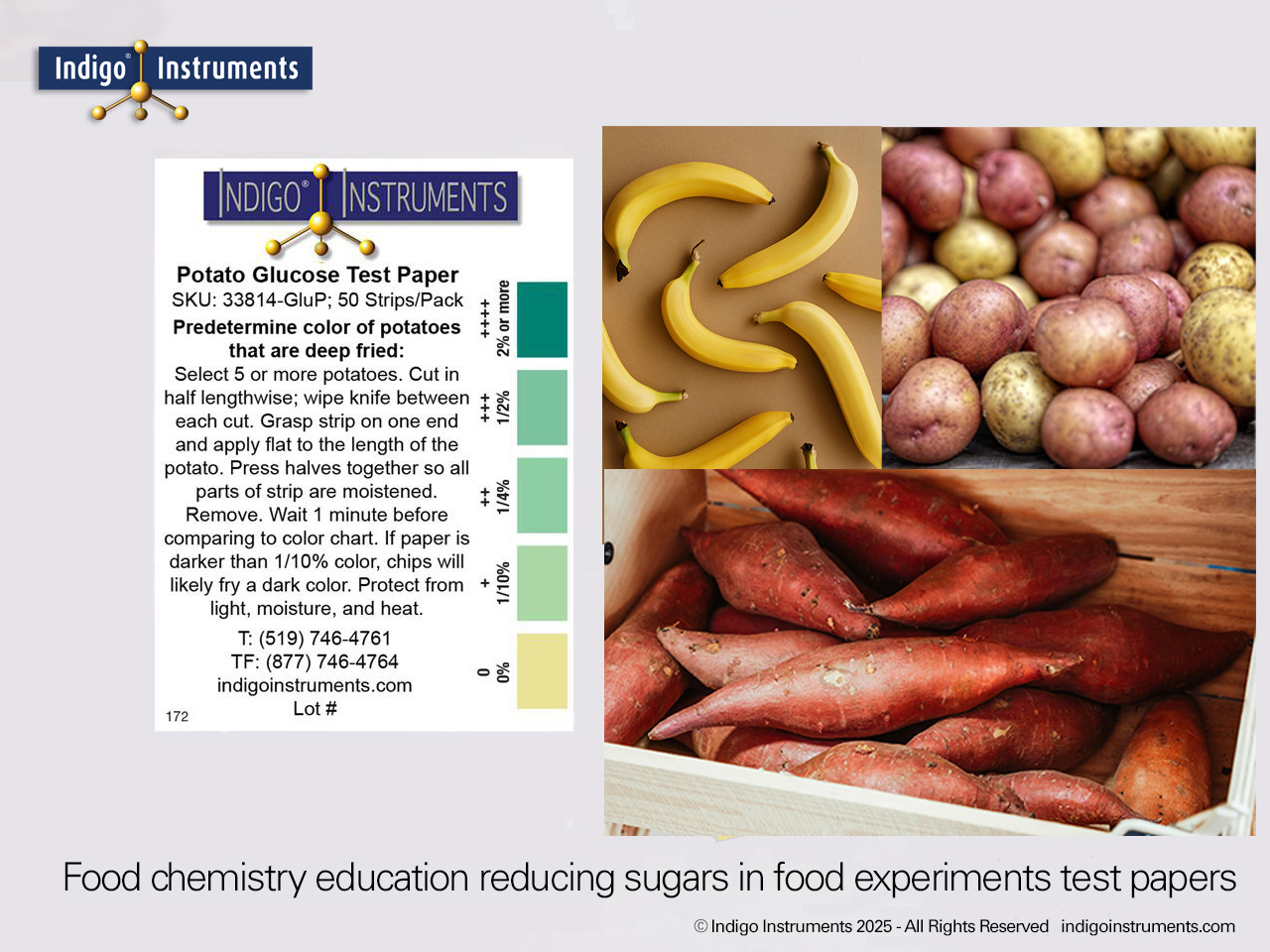
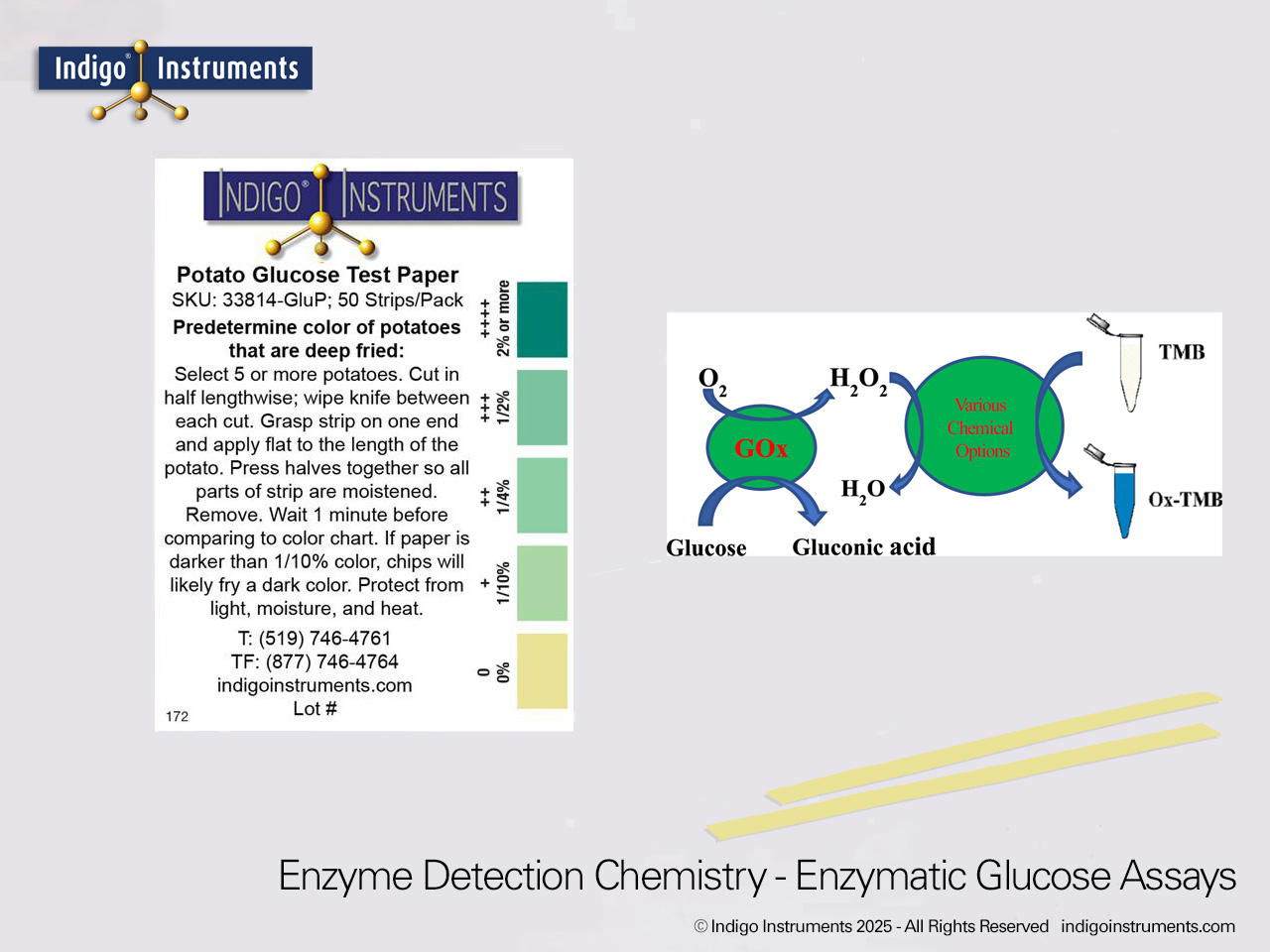
Learn how glucose and amino acids combine in the Maillard reaction to produce browning and flavor. Visualize this process using Indigo® potato glucose test paper and glucose molecular models.
The Maillard reaction is the chemical basis of browning and flavor development in foods such as bread, coffee, and fried potatoes. It begins when reducing sugars, notably glucose and fructose, react with amino acids during heating. This reaction forms a cascade of intermediates that ultimately produce melanoidins, the brown pigments responsible for desirable color and taste.
Glucose test paper provides a simple way to demonstrate how sugar concentration influences these reactions. Combined with molecular models of glucose and amino acids, it enables students to visualize the underlying chemistry behind everyday culinary transformations.