VSEPR Linear Geometry
SKU: 69180
MgCl2 (magnesium dichloride) is a molecule representative of linear VSEPR geometry & is roughly 300mm (12") long when constructed from Unit models.
The atoms in this VSEPR Large Classroom Model set can assume any geometry. Go to the bottom of page Unit Molecular Models for an overview or click on the Instructions/Safety tab to see videos on how to construct any molecular geometry.
Compare this to the model from the smaller Orbit Basic VSEPR theory set; the same structure is only 50mm (2") long.
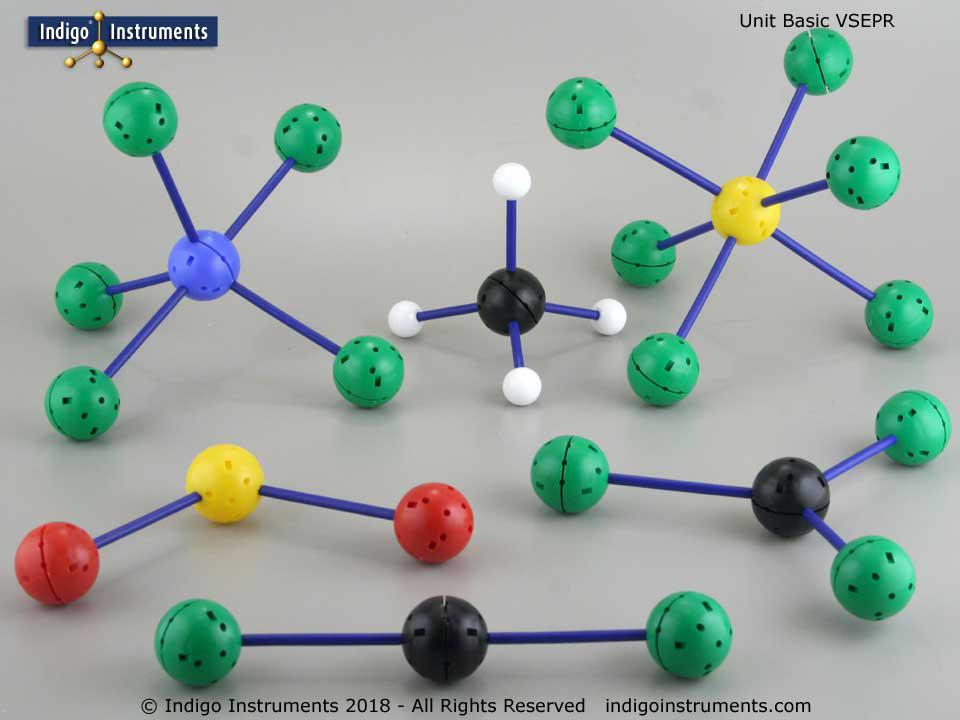
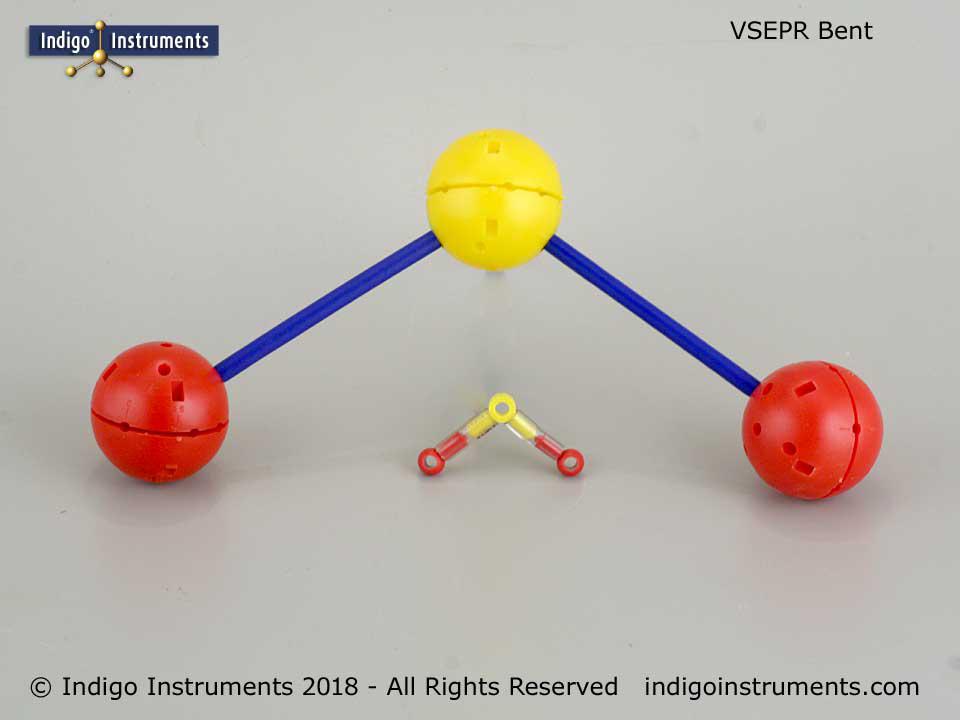
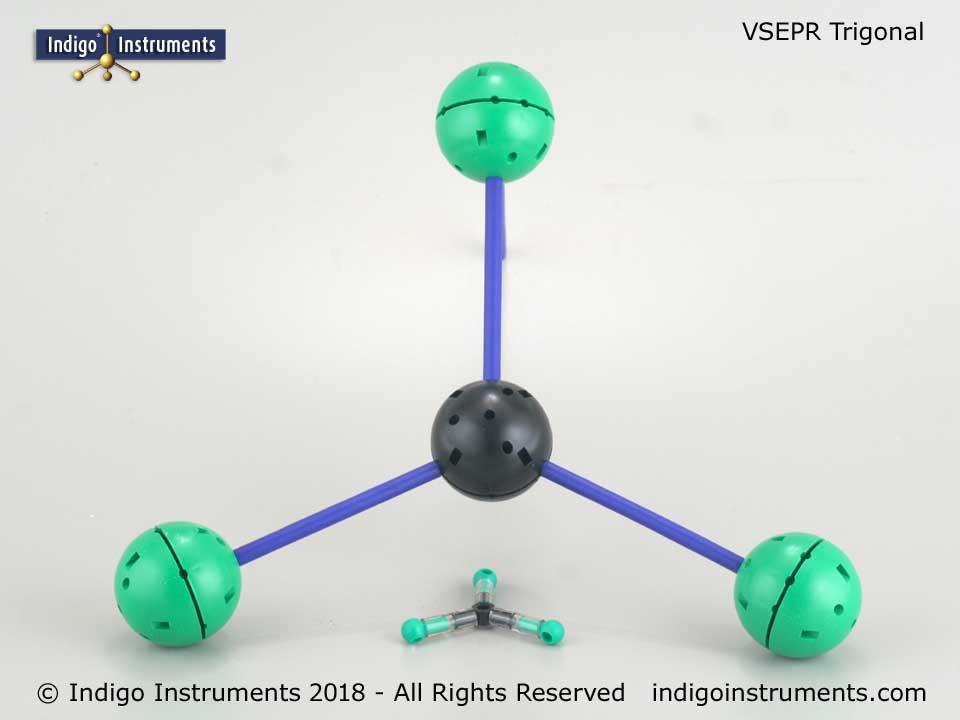
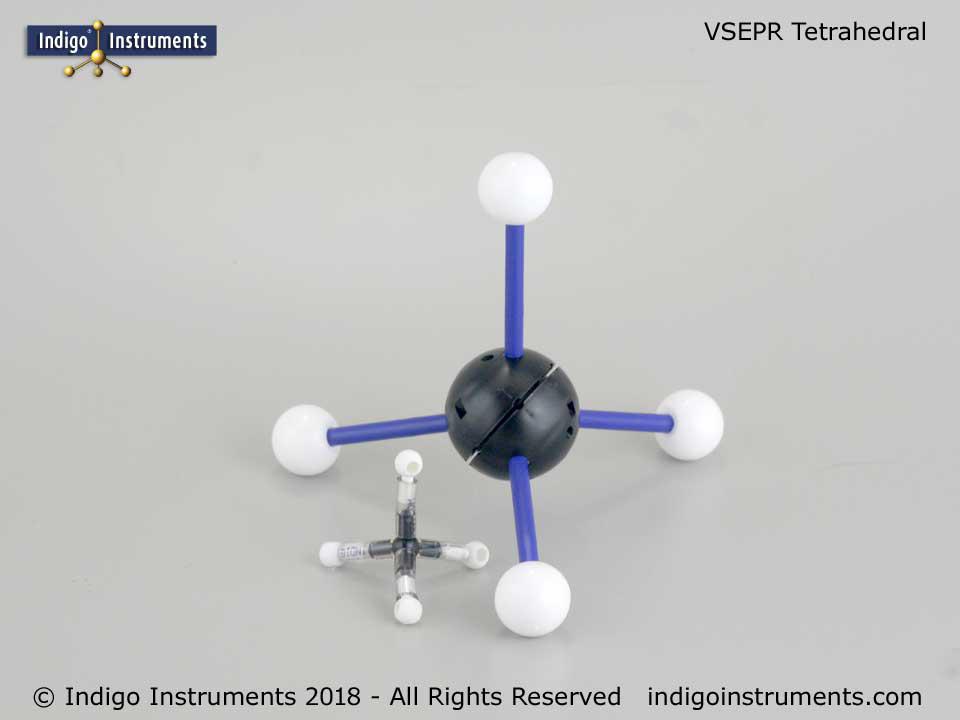
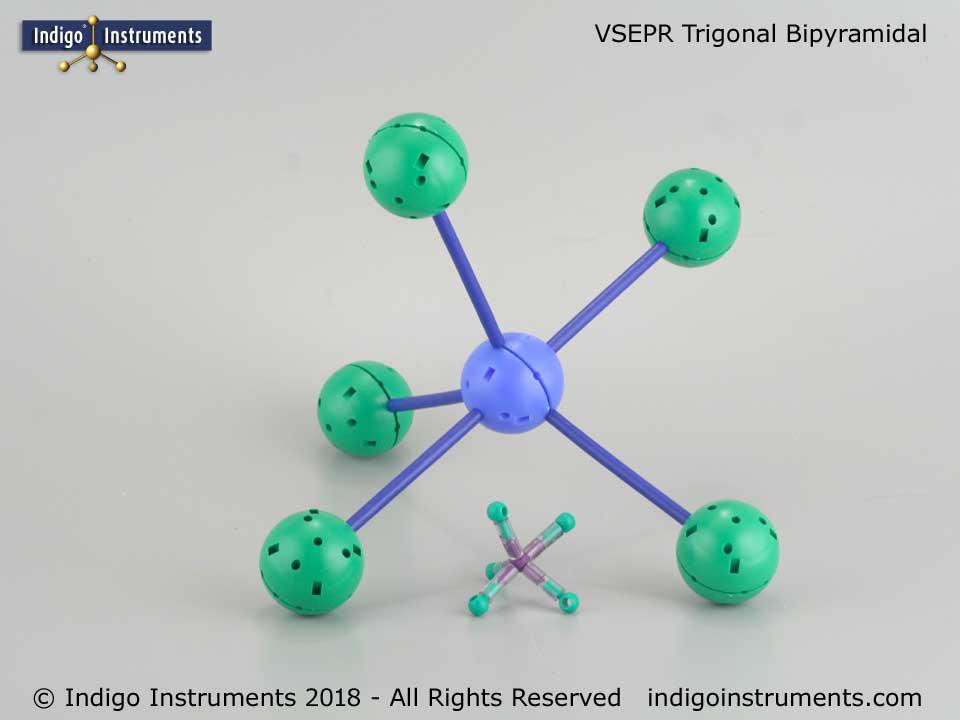
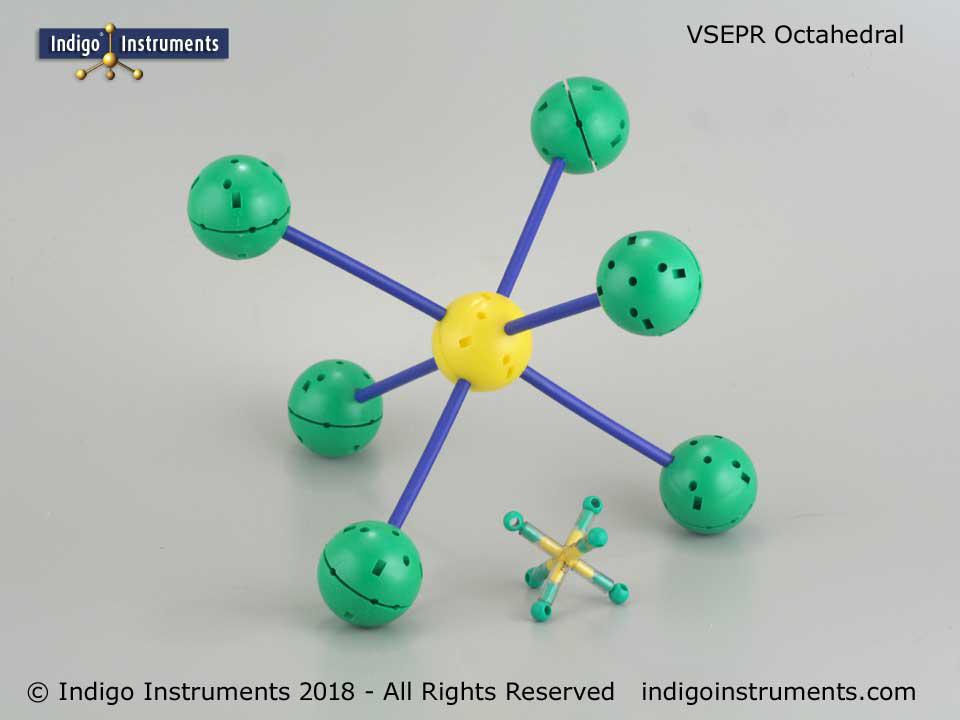
Thanks for the feedback. It can indeed be a bit tedious setting up the various hole configurations but that is also the beauty of it. No other set is as versatile in creating both standard VSEPR geometries and ones only found in crystals.