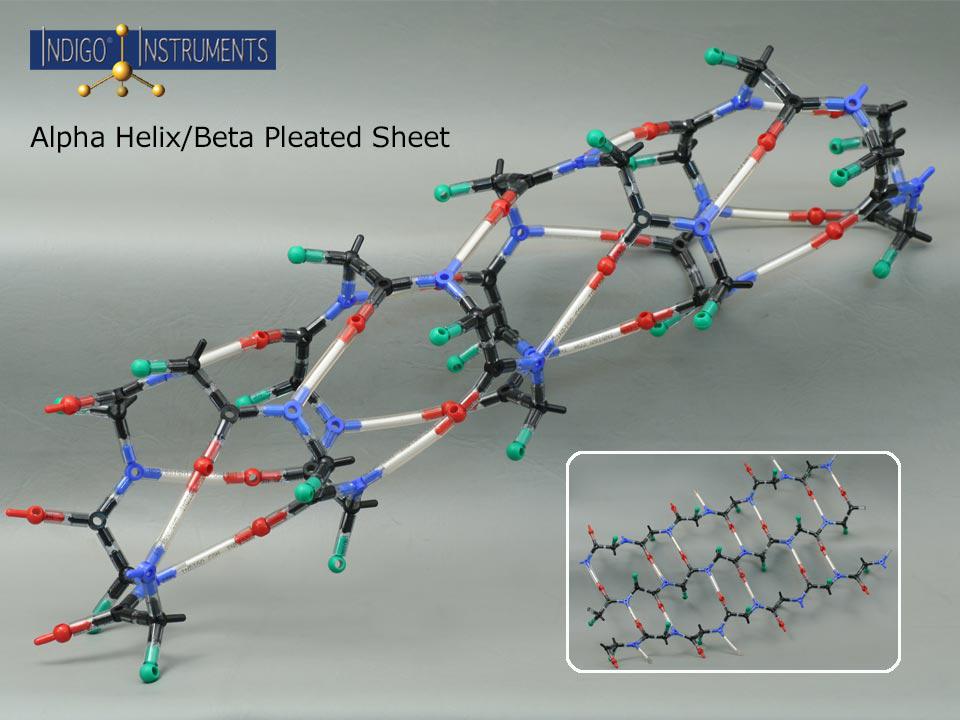
Beta Pleated Sheet
SKU: 68796W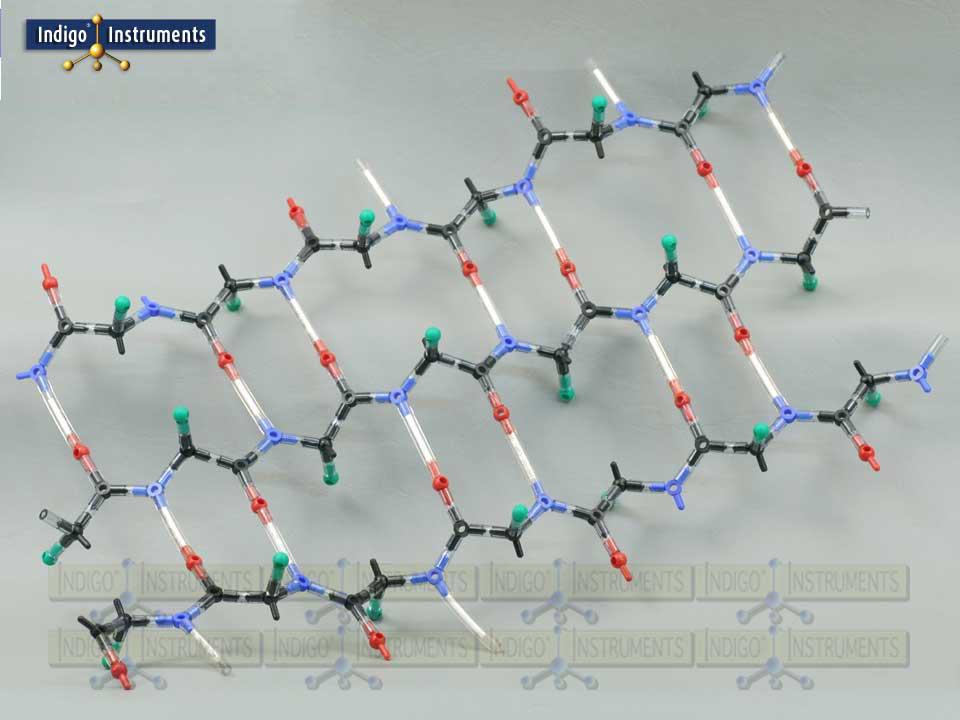
Build and explore beta pleated sheets with this Indigo® beta pleated sheet molecular model kit, visualizing parallel vs. antiparallel strands, hydrogen bonding, and side-chain orientation. Use it to explore protein secondary structure, hydrogen bonding patterns, and protein folding pathways in biochemistry.
The beta pleated sheet is one of the two principal motifs of protein secondary structure, complementing the alpha helix in influencing overall stability, aggregation potential, and protein folding pathways. This model highlights the distinctive hydrogen bonding patterns that hold beta strands together in either parallel or antiparallel arrangements. Whether used for teaching fundamentals or illustrating disease-related misfolding or biomaterial design, the hands-on experience deepens understanding of how primary sequence maps to secondary structure.
Side chains (“R” groups indicated by a green "atom") & hydrogen bonding (indicated by a 50mm clear bond with a white insert).
Indigo Instruments has maintained a substantial inventory of genuine Cochranes of Oxford (Orbit) parts for 30+ years (scroll down to see "Skeletal (Orbit/Minit) and are compatible with every molecular model kit we have sold since day 1. This level of quality may appear expensive but no parts support from other vendors costs even more.