Fluorite Crystal Mineral Structure Model
SKU: 68761W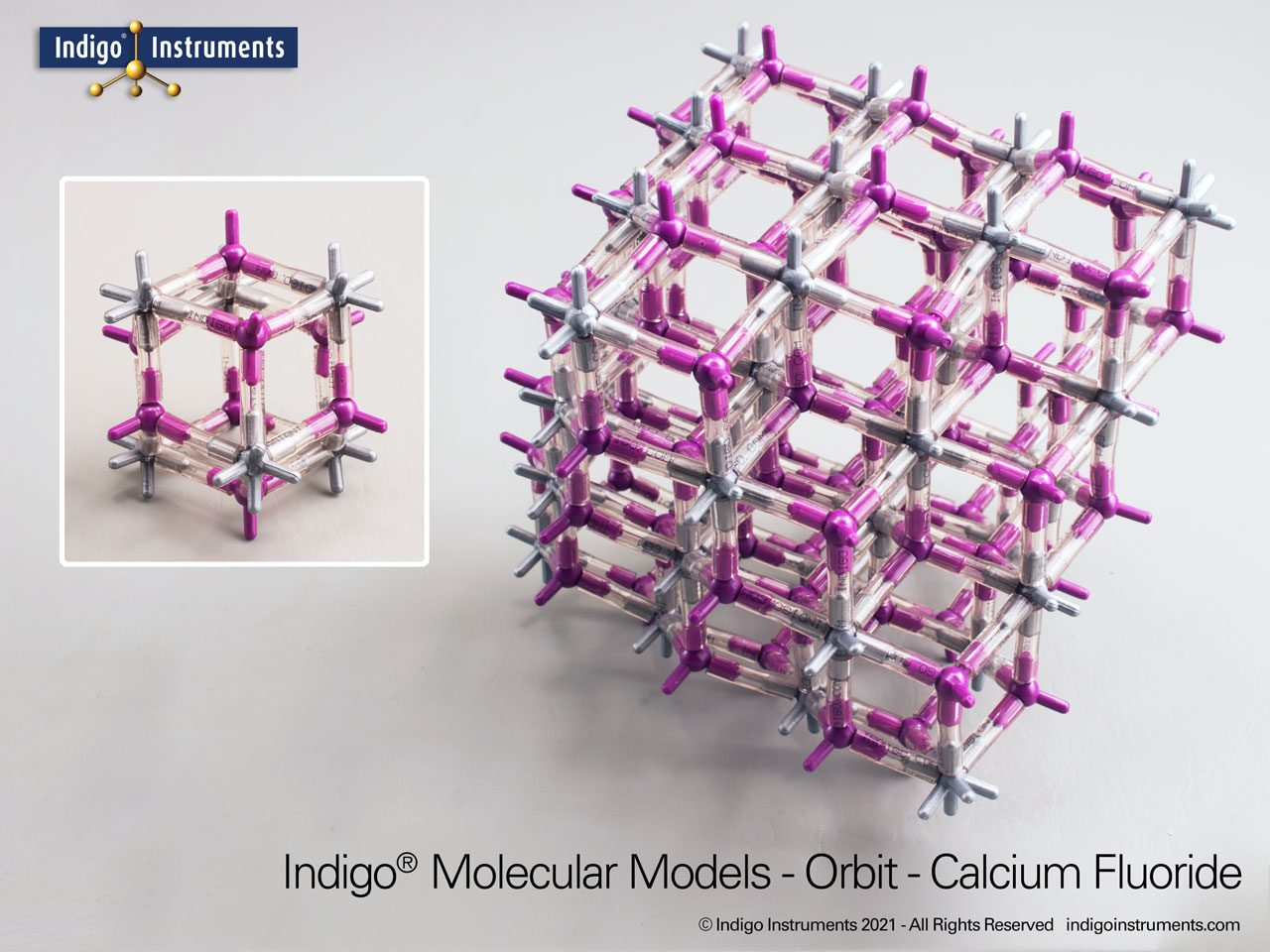
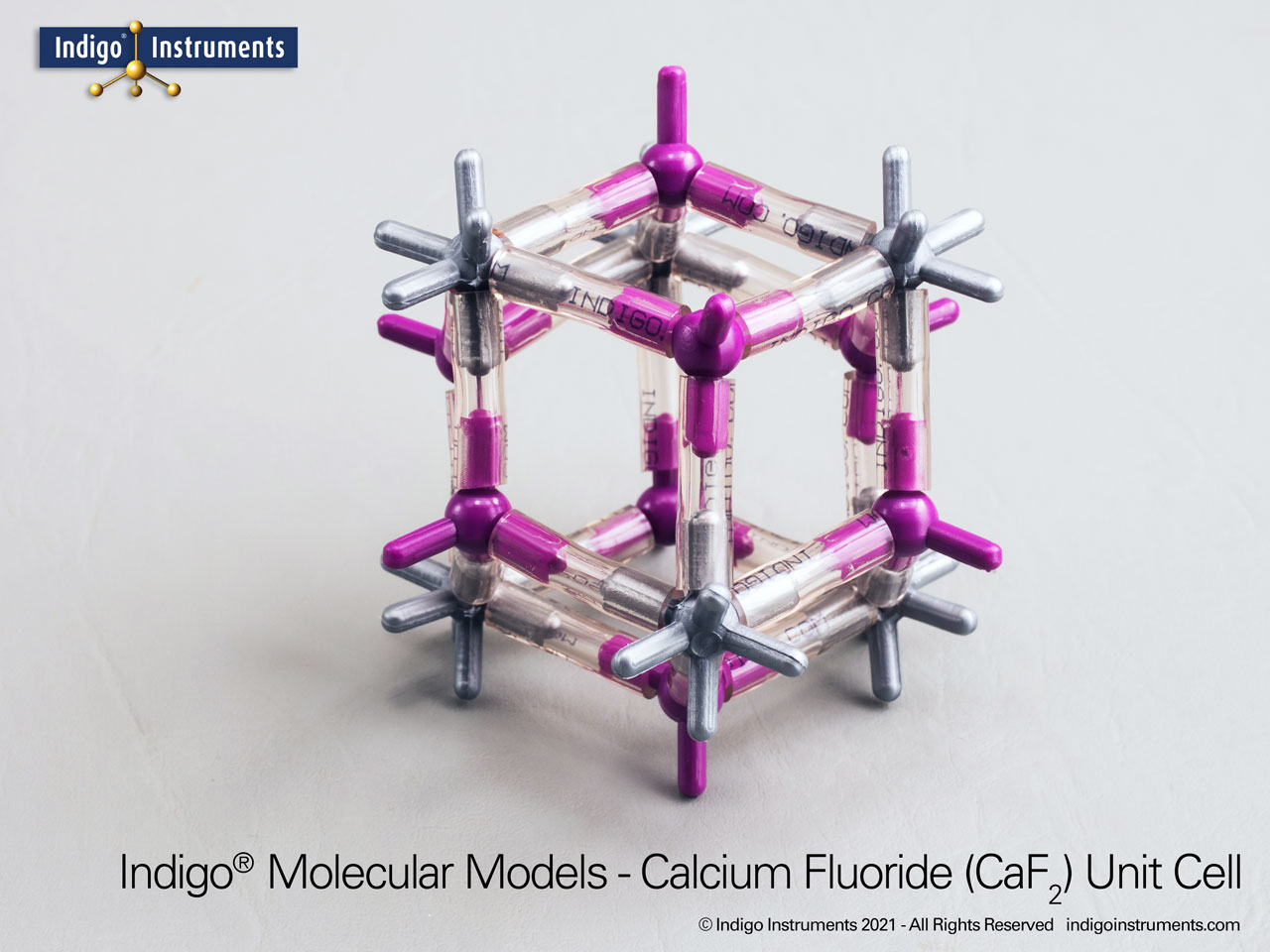
The Indigo® fluorite (CaF2) crystal lattice model comes with 154 atoms that show the 8:4 coordination, cleavage planes, and optical properties & is an ideal teaching tool for materials science & geology learning.
This fluorite crystal structure model kit displays the 8:4 coordination geometry of calcium and fluoride ions, making it a valuable tool for understanding ionic bonding, crystal lattices, and physical properties of solids. With visible cleavage planes and atomic arrangement, the model illustrates how structure influences optical behavior such as low dispersion and UV-IR transparency, as well as mechanical properties. Students, educators, and hobbyists can use it to compare lattice types, explore coordination geometry, and gain tactile insight into concepts that are often shown only in diagrams.
Indigo Instruments has maintained a substantial inventory of genuine Cochranes of Oxford (Orbit) parts for 30+ years (scroll down to see "Skeletal (Orbit/Minit) and are compatible with every molecular model kit we have sold since day 1. This level of quality may appear expensive but no parts support from other vendors costs even more.