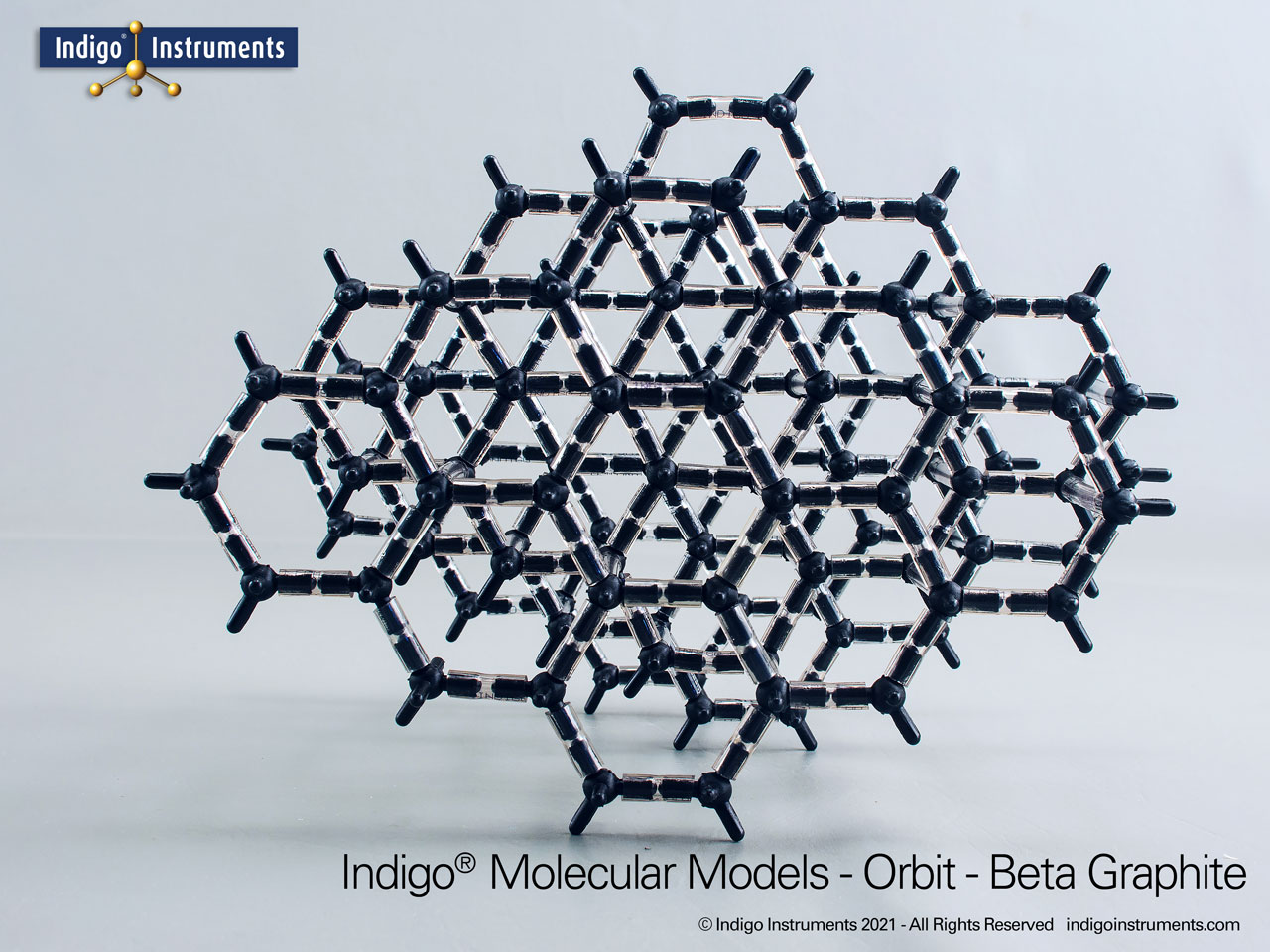
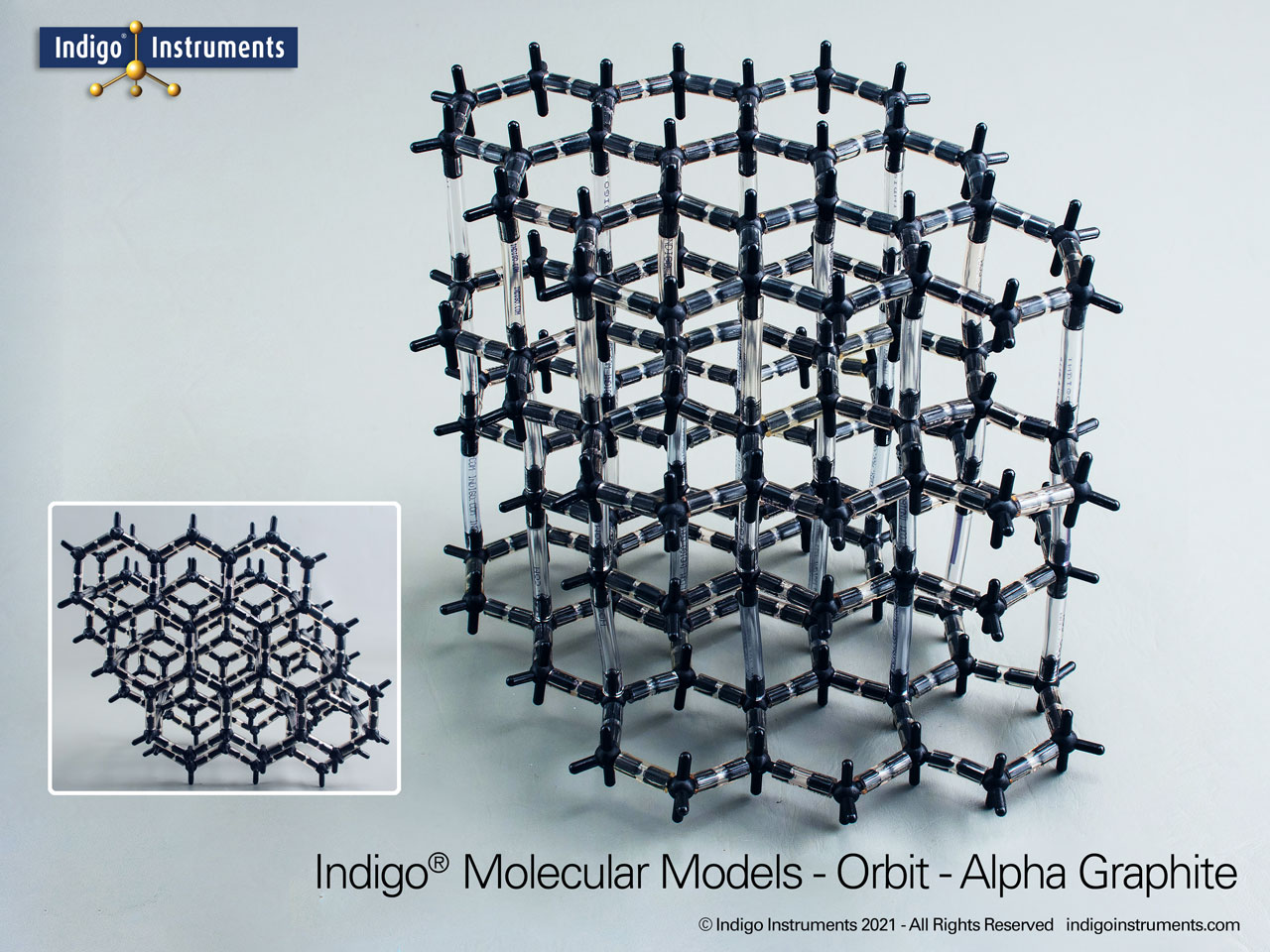
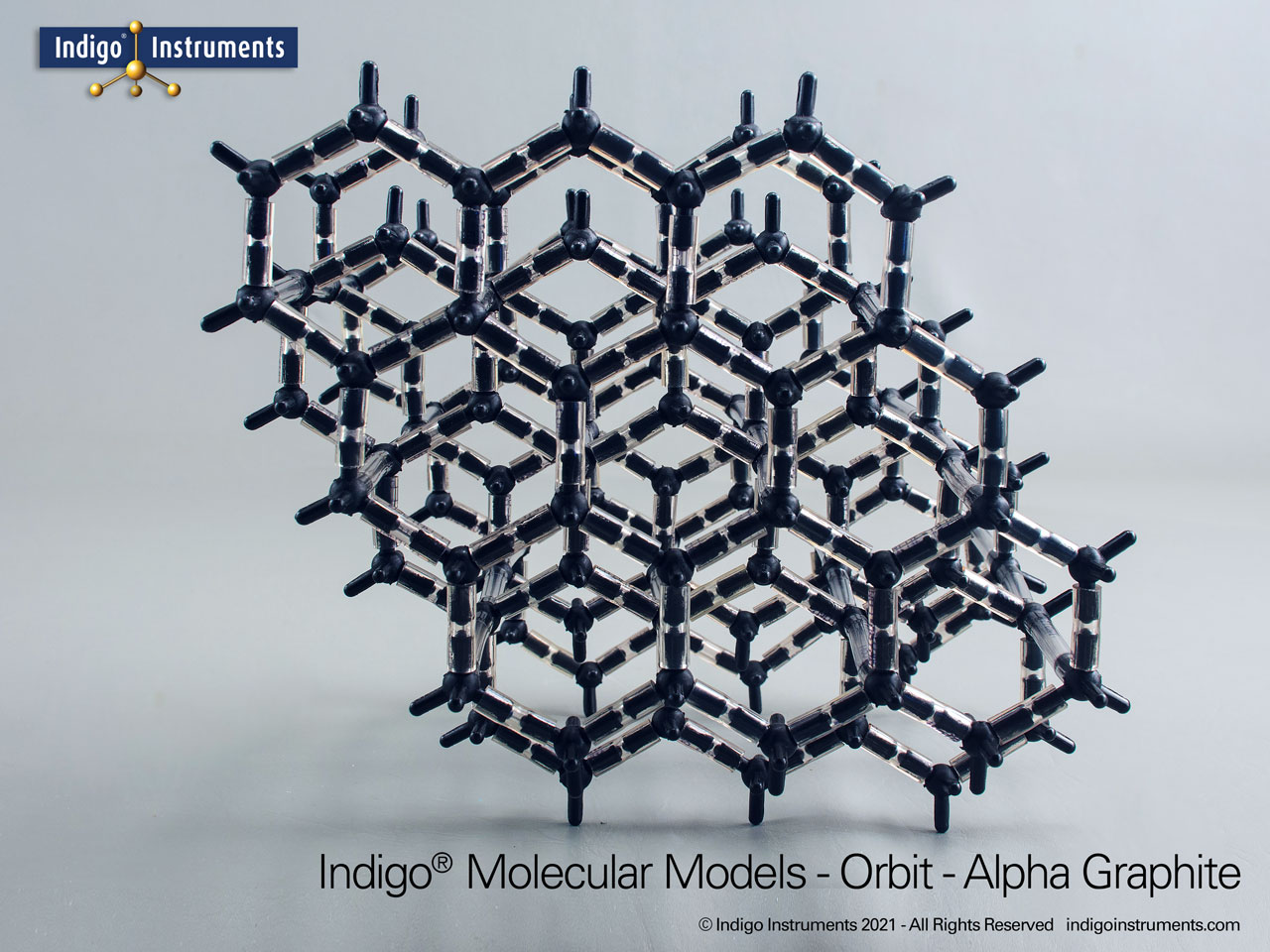
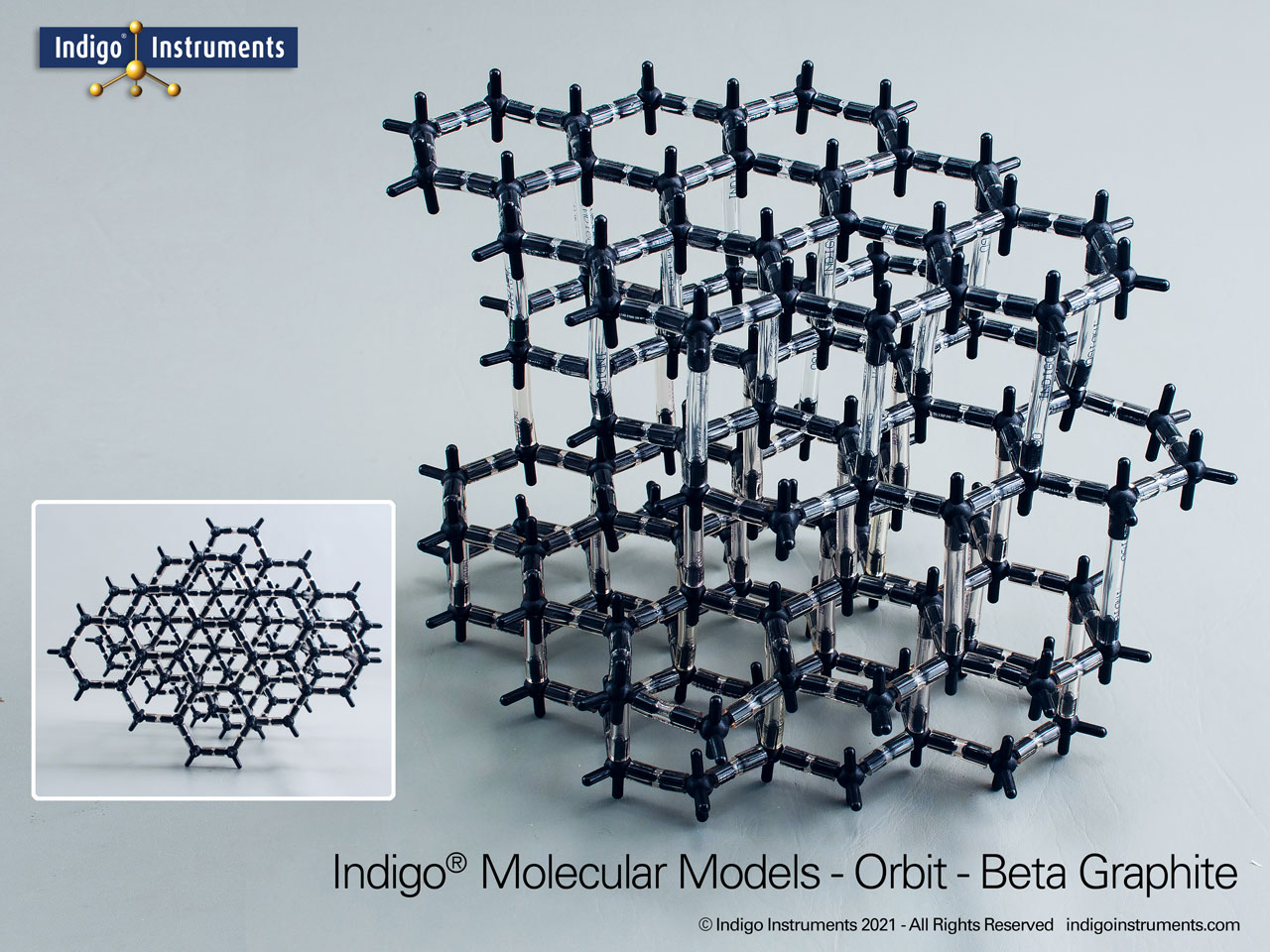
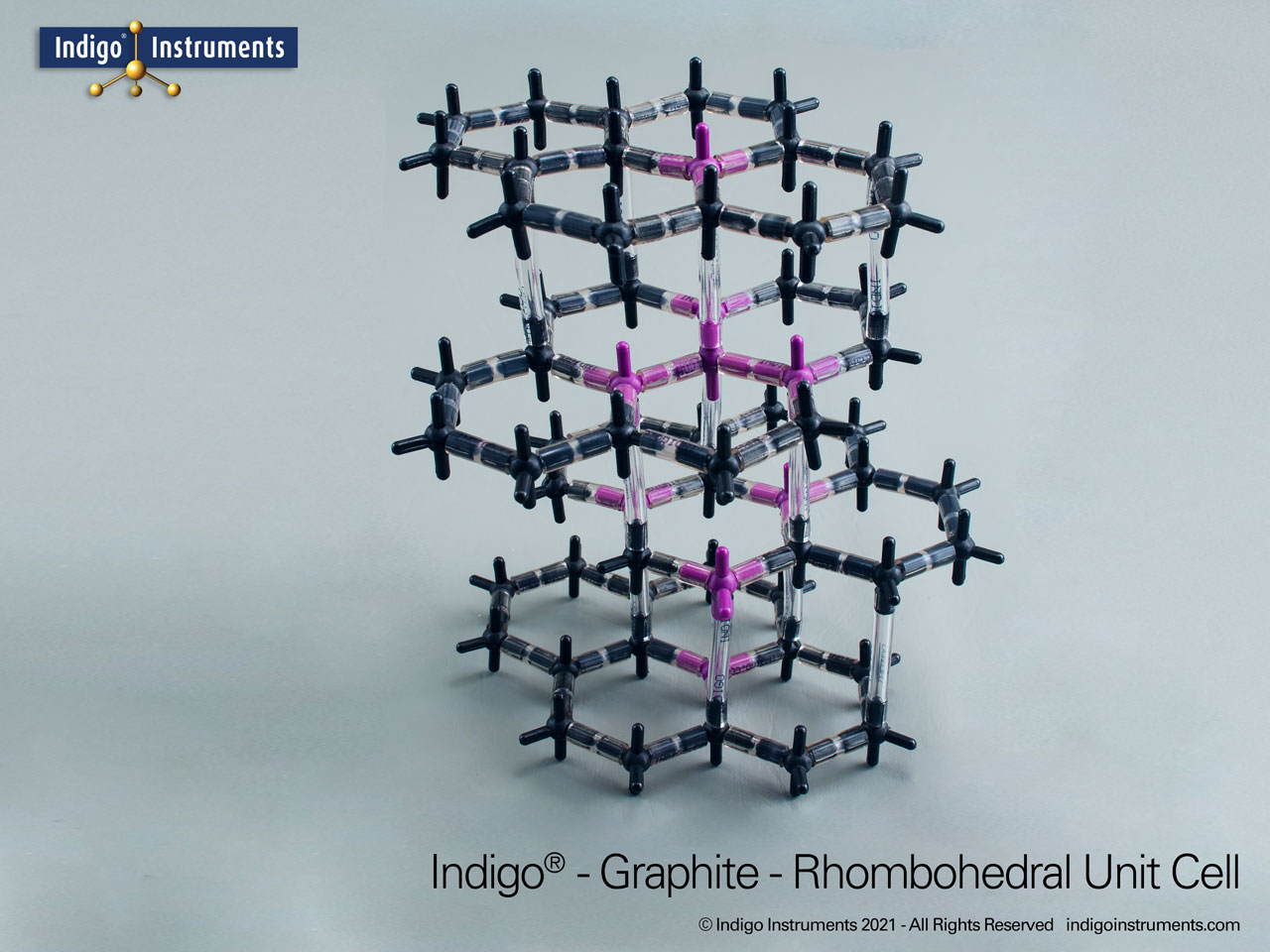
Graphite Crystal Lattice Structure Model
SKU: 68788W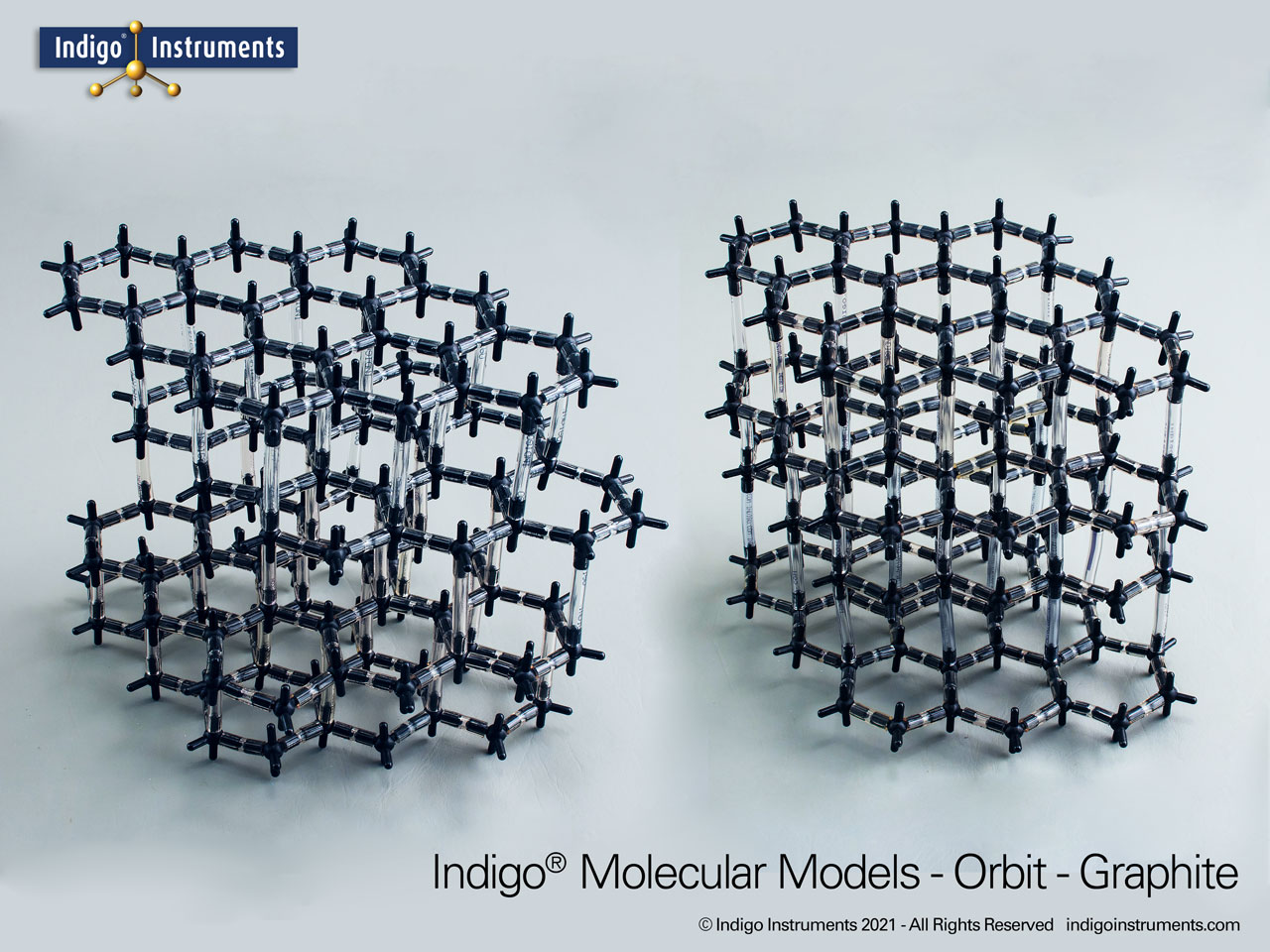
This 120 carbon atom graphite molecular model kit can build 4 layers of 6 member hexagonal rings. Each layer has the appearance of a honeycomb lattice similar to interconnected aromatic hydrocarbons.
The covalent bonded carbon atom rings use 20mm bonds while inter-layer Van der Waals atoms are supported with 50mm bonds. Hold the top & bottom of the model & squeeze in opposite directions. You can show how the layers slide against other & how pencil lead works. Stiffer 10cm versions of these bonds can be used with trigonal atoms but to lesser effect.
Build as the more common alpha-hexagonal (above right) with ABAB layer stacking or the beta-rhombohedral (above left) ABCA layering. Details below.
Indigo Instruments has maintained a substantial inventory of genuine Cochranes of Oxford (Orbit) parts for 30+ years (scroll down to see "Skeletal (Orbit/Minit) and are compatible with every molecular model kit we have sold since day 1. This level of quality may appear expensive but no parts support from other vendors costs even more.