Lead Acetate Hydrogen Sulfide Test Paper
SKU: 33810-PbAc
Detect hydrogen sulfide and other reduced sulfur compounds that release H2S gas in air, water, or solid materials with lead acetate test paper. Nominally sensitive to ~5 ppm H2S, it is ideal for corrosion control, environmental testing, and air-quality monitoring.
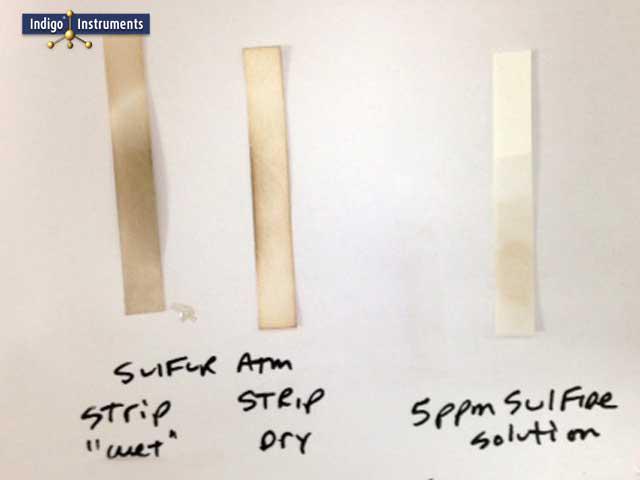
Lead acetate test paper provides a simple, rapid means of detecting hydrogen sulfide (H2S) and related sulfur-bearing gases that cause corrosion or tarnish. When exposed, the white paper darkens to gray or black as lead(II) acetate reacts with H2S to form lead sulfide (PbS).
The test paper is nominally sensitive to ~5 ppm H?S under favorable conditions. Lower concentrations may still produce faint darkening after longer exposure, but results become increasingly subjective. For this reason, these papers are best described as qualitative or semi-quantitative indicators rather than instruments for precise measurement. Simply moisten the strip for exposure to air or dip in suspect liquid. The reaction is time dependent, higher H2S levels darken the paper rapidly, while trace levels may take several minutes.
Hydrogen sulfide gas is lethal in relatively low concentrations. If you smell it & then you don't, play it safe & leave immediately! You also might find this article on gas stove leaks interesting.


Thanks for the kind words & great review. We knew how important these strips were to your business & we happy to nip it in the bud!